The structure of steroids fundamentally affects their effect at the N-methyl-D-aspartate receptors
N-methyl-D-aspartate receptors (NMDARs) are proteins involved in the regulation of many processes in the mammalian brain. They play a key role in signal transmission between nerve cells, and even a small disturbance in the NMDAR function may have sever pathophysiological effects. A reduced function of NMDARs is associated with neuropsychiatric disorders such as mental retardation, reduced intellect, schizophrenia, autism spectrum disorders, epilepsy, or motor disorders. The insufficient NMDAR function can be compensated for by numerous compounds, including neurosteroids. However, the mechanism by which neurosteroids potentiate NMDARs is not well understood.
In a study published in the British Journal of Pharmacology, we investigated the effect of newly synthesized synthetic analogues of endogenous neurosteroid pregnanolone sulfate on NMDARs. We demonstrated that analogues with short aliphatic chains, such as pregnanolone carboxylate (PA-Car), inhibit NMDAR responses, whereas analogues with longer aliphatic chains, such as epipregnanolone butyrate (EPA-But), potentiate NMDAR responses. Combining electrophysiology, molecular biology, and computational modelling, we identified the binding site for EPA-But at the transmembrane domain of NMDAR and suggested the mechanism by which EPA-But enhance the NMDAR function.
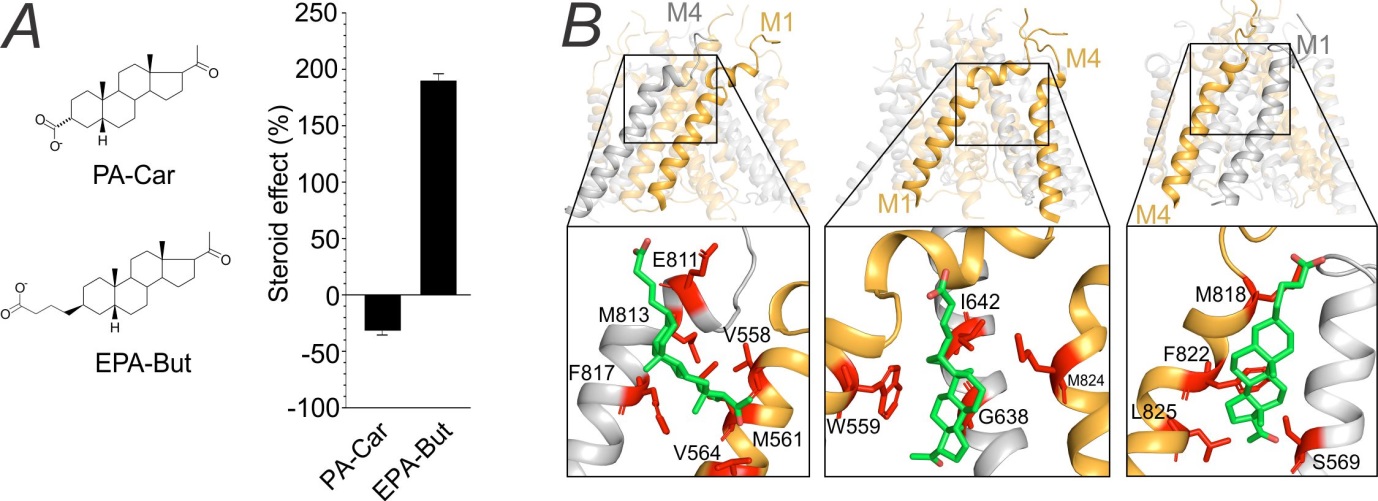
(A) Left, structure of pregnanolone carboxylate (PA-Car) and epipregnanolone butyrate (EPA-But). Right, graph shows the average effect of PA-Car and EPA-But at NMDAR. (B) Location of the EPA-But binding site at the transmembrane domain of NMDAR. Amino-acid residues that are involved in the interaction with EPA-But molecule (green) are highlighted in red.
Kysilov B, Hrcka Krausova B, Vyklicky V, Smejkalova T, Korinek M, Horak M, Chodounska H, Kudova E, Cerny J, Vyklicky L: Pregnane-based steroids are novel positive NMDA receptor modulators that may compensate for the effect of loss-of-function disease-associated GRIN mutations. British Journal of Pharmacology, (2022) 179:15, 3970–3990, IF = 9.473, DOI
