Creation of new tissue replacements
The general principle of the creation of tissue replacements, artificial material containing as an analogue of extracellular matrix (ECM) and the cell
The material thus constructed tissue replacement is a resorbable, ie. will be gradually eliminated and replaced with regenerated tissue. So artificial material in modern tissue engineering becomes not permanent replacement of damaged tissues, but only a temporary carrier cells inducing the regeneration of damaged tissue. The concentration and distribution of adhesion oligopeptides, i.e. ligands for adhesion cell receptors, regulates the number and the spreading area of the adherent cells. The amino acid composition of the oligopeptide then determines the type of the adhered cells. E.g. the REDV sequence (Arg-Gly-Asp-Val) is preferred by endothelial cells, the sequence VAPG (Val-Ala-Pro-Gly) by the smooth muscle cells, the sequence KRSR (Lys-Arg-Ser-Lys) by the osteoblasts and the sequence RGD (Arg-Gly-Asp) is for all cell types.
In our studies we used the mentioned model for tissue engineering of blood vessel wall. The polymeric material with oligopeptid ligands containing RGD (the ligands for the integrin adhesion receptors) was seeded with vascular smooth muscle cells, which are the most abundant cell type of the vessels, providing especially their contractility.
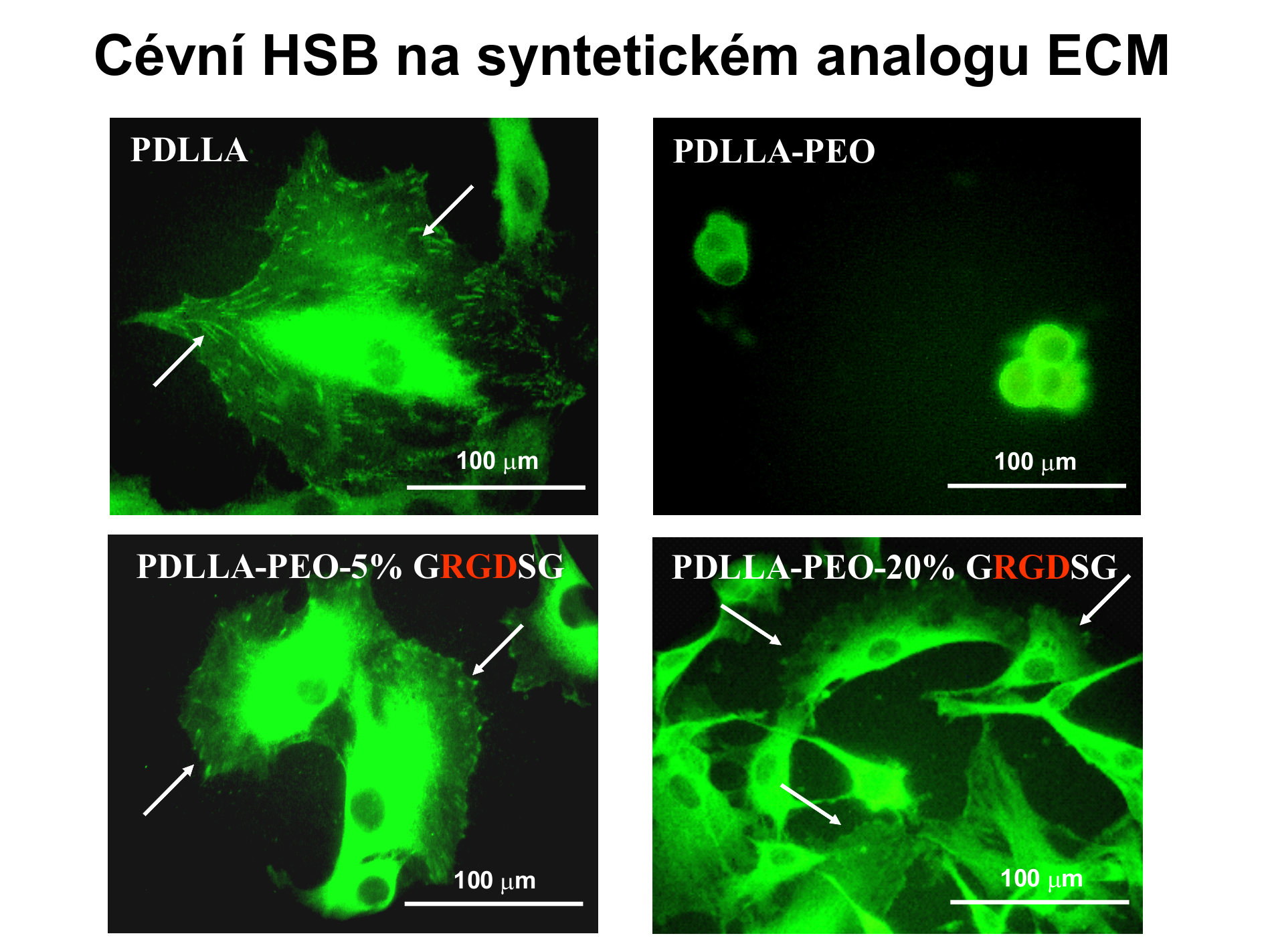
In the culture on the polylactide (PDLLA) supplemented with the medium containing fetal bovine serum are the smooth muscle cells (HSB) normally spread and have a polygonal shape, similarly as is in the cultures from the standard polystyrene cultivation dishes. The cells have a well-formed focal adhesion plaques (arrowed),i.e. group of adhesion receptors and other associated molecules, through which the cells adhere to the substrate. Cell adhesion to the polylactide is mediated by spontaneous adsorption of proteins from the serum (especially vitronectin and fibronectin), which is a cell adhesion receptors bind.
If the polylactide chain copolymerized with polyethylene oxide chains (PEO-PDLLA), the surface of the material becomes strongly hydrophilic and mobile. Mobility can be imagined such as that chain PEO "flutter" in the culture medium, like grass or grain ropes in the wind. This eliminates adsorption of proteins mediating the adhesion of cells from serum in the culture medium and the cells can not adhere to the substrate. They have a spherical shape and the lack of cell-adhesion material is trying to compensate with the cell-cell adhesion, i.e. by creating clusters.
If some chain of PEO (in this case 5 or 20%) fitted with adhesion oligopeptides containing RGD (PDLLA-PEO-5% GRGDSG or PDLLA-PEO-20% GRGDSG), cell adhesion, their distribution on the substrate and formation of focal adhesion plaques are restored. The concentration of adhesion ligands affected the surface spreading of cells and the number of adherent cells in the sense that the spreading area was higher at lower concentrations of ligands, whereas the number of cells was proportional to the concentration of ligands.
This principle of the creation of tissue replacement could be used in general for engineering a wide range of tissues, including bone tissue. Polymeric materials are generally less suitable for replacements of bone tissue for its poor mechanical properties. Therefore we attempted to reinforce polymers with harder materials such as nanoparticles of diamond or ceramic materials, e.g. hydroxyapatite. These nanoparticles represent an inorganic (mineral) component of natural bone tissue. Polymers are used in the form of nanofibres, which mimicthe organic components of natural bone tissue, e.g. collagen fibers.
Potential substitutes of bone tissue based on polymer nanofibers reinforced with organic particles
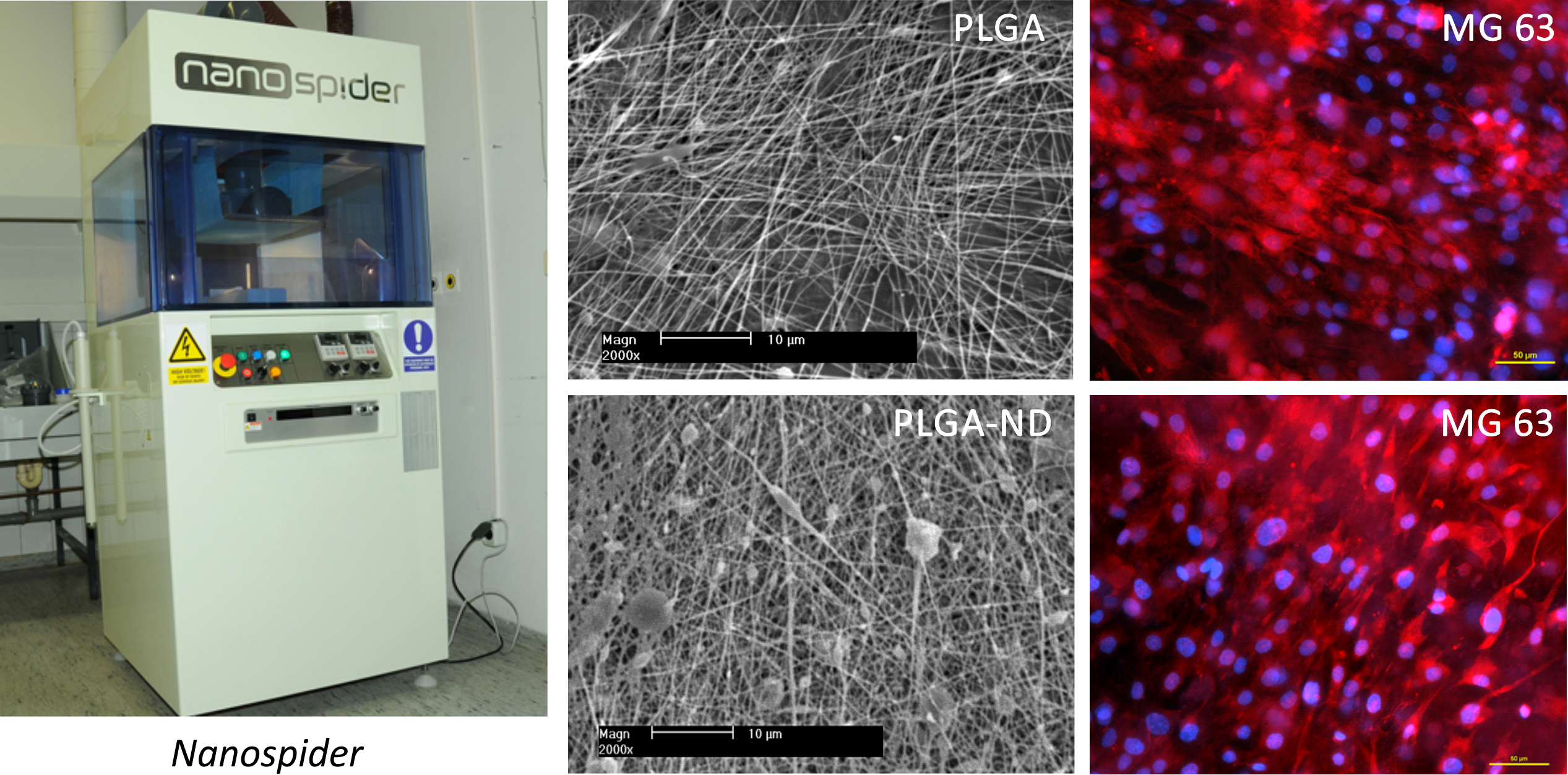
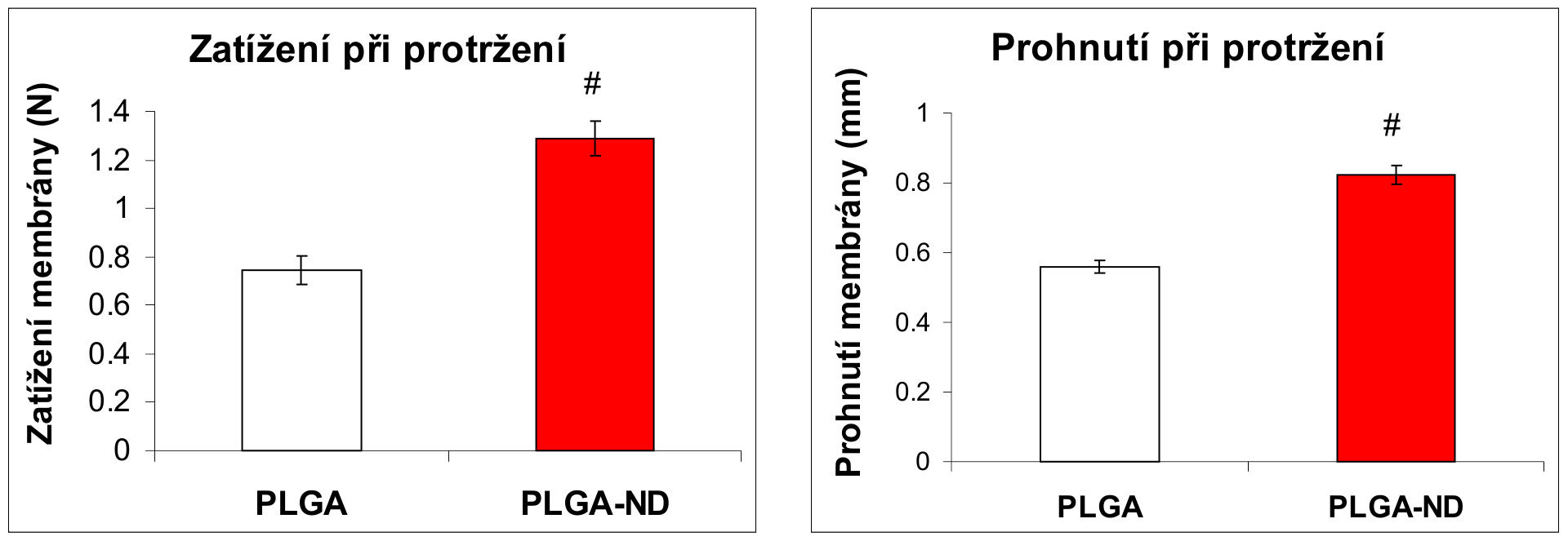
Nanofibrous membranes from the copolymer of lactide and glycolide (PLGA) were prepared by the electrostatic spinning method in the NanospiderTM device (Elmarco Ltd.). Some membranes have been loaded with diamond nanoparticles of diamond, admixed into the PLGA solution before spinning (PLGA-ND). The final concentration of nanodiamond in PLGA was about 23 wt%. Human osteoblasts line MG 63 adhered and grew on both types of scaffolds similary, but the PLGA-ND membranes showed higher mechanical resistance, as demonstrated by rupture tests of load and deflection of rupture probe at failure.
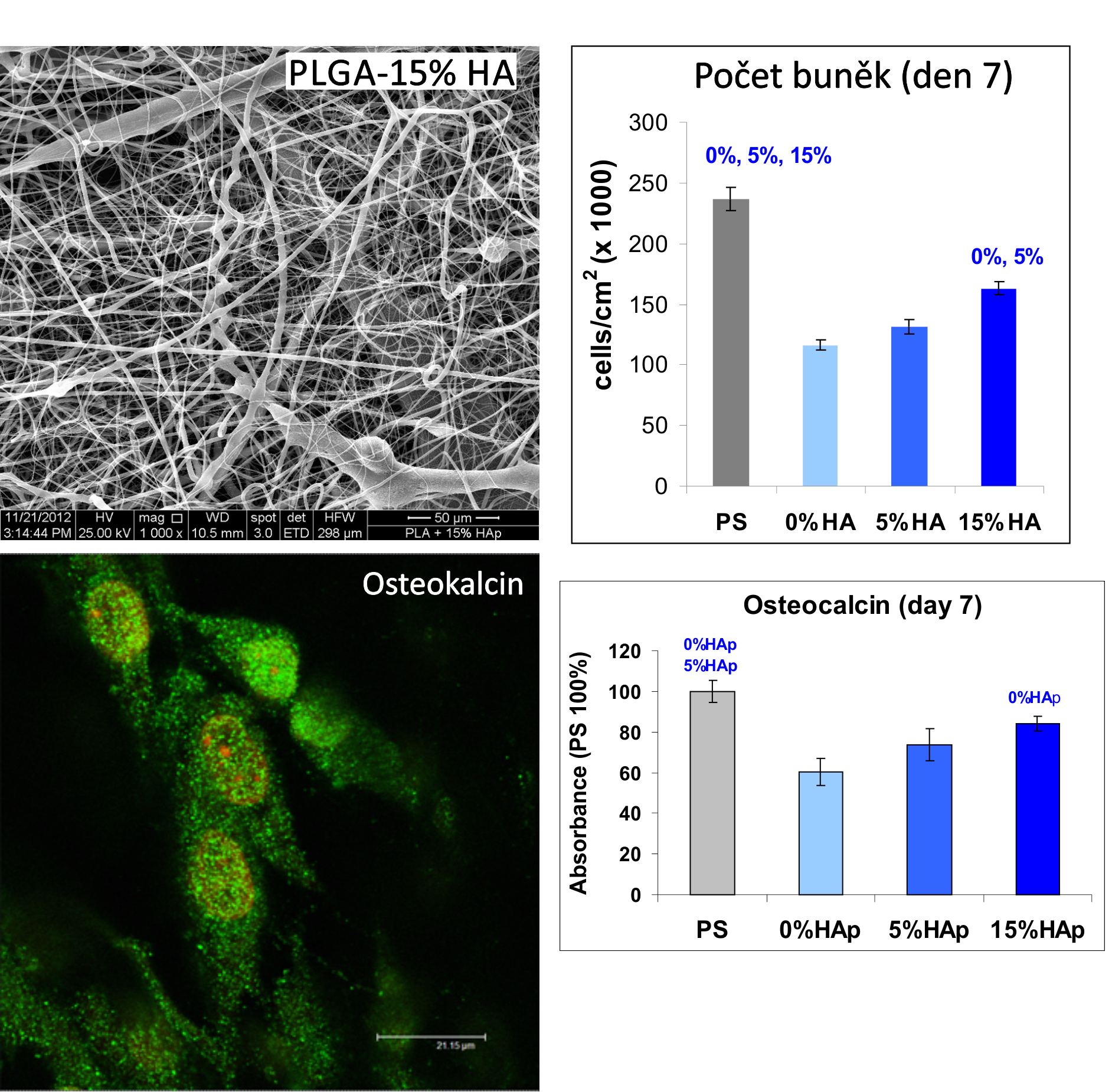
Positive results were also achieved with nanofibrous membranes fabricated from the polylactide (PLLA) loaded with 5 and 15% by weight of hydroxyapatite (HA). The presence of HA supported the growth of osteoblasts line MG 63, and also their osteogenic differentiation which manifested a higher concentration of osteocalcin in these cells: