Special techniques to estimate cell physiological parameters
Measurement of intracellular pH
For intracellular pH measurements, we use pHluorin (a green fluorescent protein sensitive to changes of pH). A gene encoding pHluorin is introduced into yeast cells either on plasmid or it is integrated into the genome. The method enables intracellular pH measurements in yeast cells upon physiological conditions. Principle: pHluorin has two excitation maxima at 395 and 475 nm. The intensity of pHluorin fluorescence at these wavelengths depends on concentrations of protons (pH). We determine the ratio of fluorescence intensities at excitation maxima and a pH value is calculated from a calibration curve (=dependency of fluorescence intensity 395 nm/475 nm ratio in cells expressing pHluorin on pH of calibration buffers).
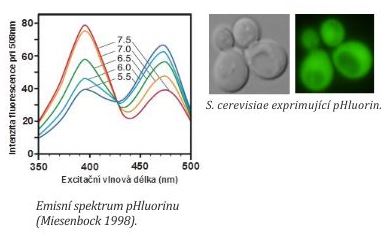
For intracellular pH measurements, we use a fluorescent reader for the determination of internal pH value in cells in cells suspensions. Together with the laboratory of Jiří Hašek at the Institute of Microbiology AS CR, v.v.i. and the DEL Company we developed a software application Ocellaris that enables detection of cells in the microscope and intracellular pH measurements at the single cell level. For these measurements, we use the Cellasic system.
Intracellular cation content measurements
Studying the properties and physiological roles of alkali-metal-cation transporters, we are often interested, how the presence, absence or changes structure of transporters influences intracellular cation concentrations. To estimate the cation content in cells, the cells are extracted with hydrochloric acid and cation concentrations are measured in the extracts using flame atomic absorption spectrometry.
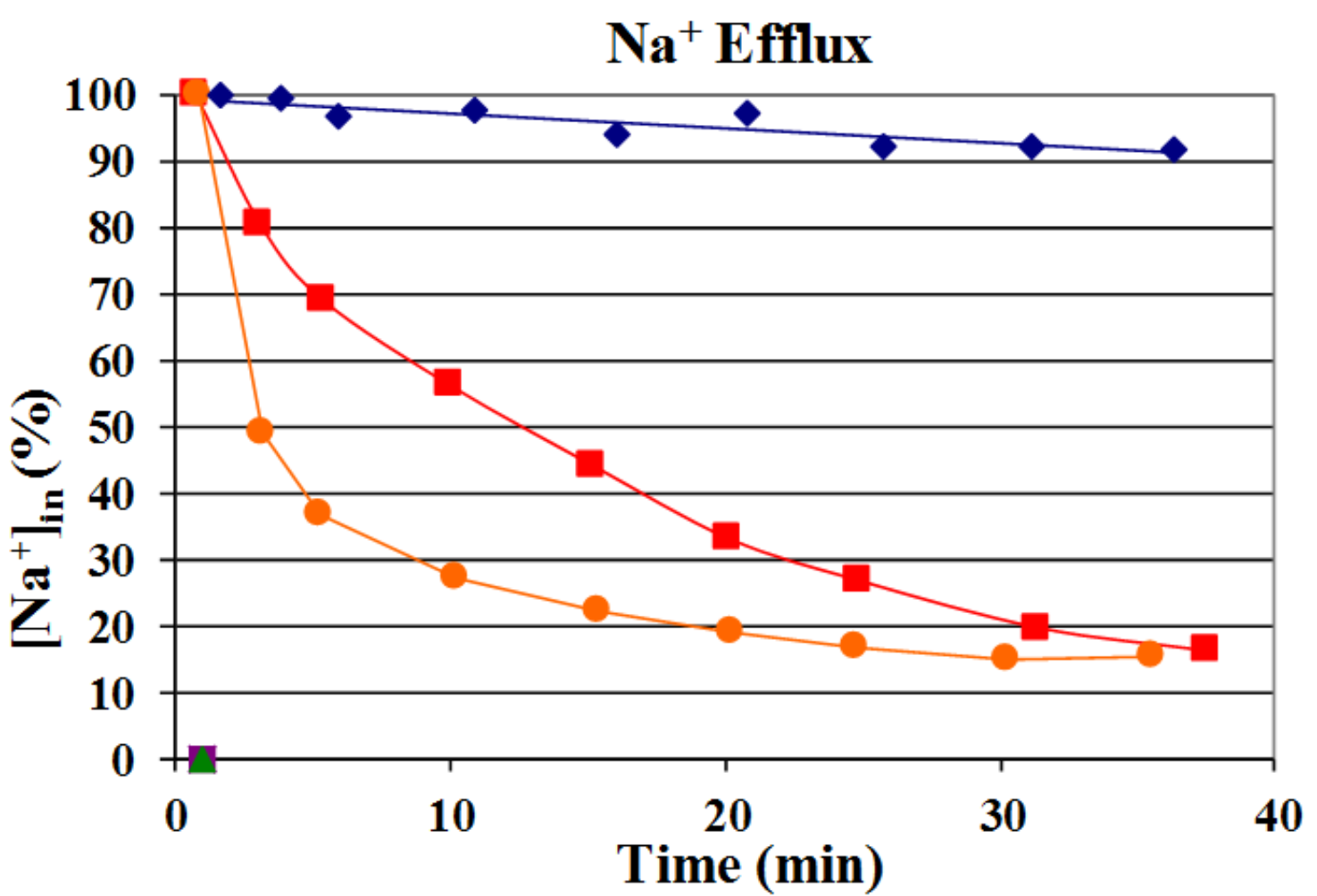
Estimation of sodium content within 40 min in S. cerevisiae BW31 cells without sodium exporters (blue ♦) or producing Sc Nha1p (red ■) or Yl Nha2p (orange ●) transporters shows that YlNha2 is more effective (exports Na+ faster) than ScNha1.
Measurement of relative membrane potential
Measurement of plasma-membrane potential is based on the use of the diS-C3(3) fluorescence probe (Gášková et al., 1998). The probe’s maximum of fluorescence emission and intensity of fluorescence respond to changes in membrane potential of cell suspension. This cationic probe easily enters into the cells and accumulates inside proportionally to the inside-negative membrane potential until equilibrium is reached. Cells with a high membrane potential (hyperpolarization of plasma membrane) accumulate more probe, in contrast to depolarized cells that contain lower probe amounts.
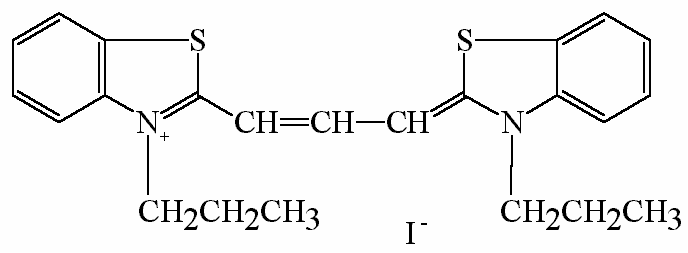
diS-C3(3) (3,3´-dipropylthiacarbocyanine iodide)
Fluorescence response of diS-C3(3) to membrane potential is significantly influenced by the activity of MDR pumps. The probe is expelled from the yeast S. cerevisiae via ABC pumps Pdr5 a Snq2 and from C. glabrata via Cdr1, therefore it is possible to use it for the study of activity of MDR pumps and their putative inhibitors.
Principle:
The binding of the probe diS-C3(3) to the cell components is accompanied by changes in its spectroscopic properties:
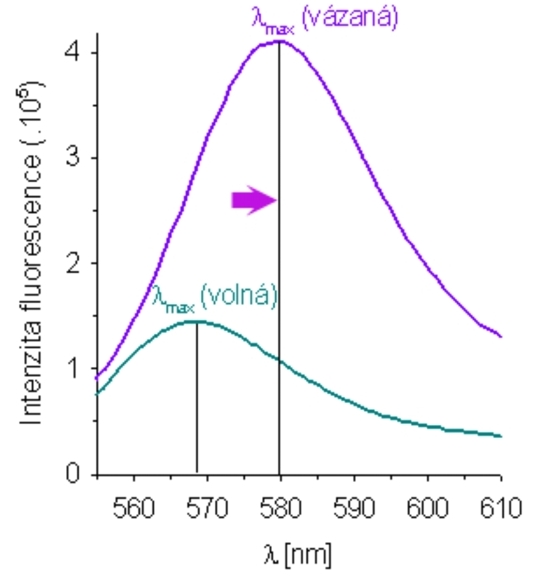
- Maximum of fluorescence emission of bound probe is shifted towards longer wavelengths (~ 10 nm) in comparison to maximum of emission of free probe (569 nm).
- Intensity of fluorescence considerably increases due to longer lifetime and bigger quantum yield.
The method is based on monitoring of the time dependence of the position of maximum emission after addition of the fluorescence probe to the cell suspension, the so-called staining curve.