Structural basis of the 14-3-3 protein-dependent activation of ubiquitin ligase Nedd4-2
Our results show the the structural glimpse on the 14-3-3-dependent regulation of human ubiquitin ligase Nedd4-2. Our data could be important for understanding of the regulation process of Nedd4-2 as well as of the role of 14-3-3 proteins in the regulation of other ubiquitin ligases.
Our research team has been studying the 14-3-3 proteins which are highly conserved regulatory molecules found in all eukaryotes. First they have been isolated from the bovine brain and their unusual name “14-3-3”, originates from their elution and migration pattern on two-dimensional DEAE-cellulose chromatography and starch gel electrophoresis. 14-3-3 proteins have the ability of binding the functionally different signal proteins, including kinases, fosfatases and transmembrane receptors by changing their function. Through the functional modulation of a wide range of binding partners, 14-3-3 proteins are involved in many processes, including cell cycle regulation, metabolism control, apoptosis,and control of gene transcription.
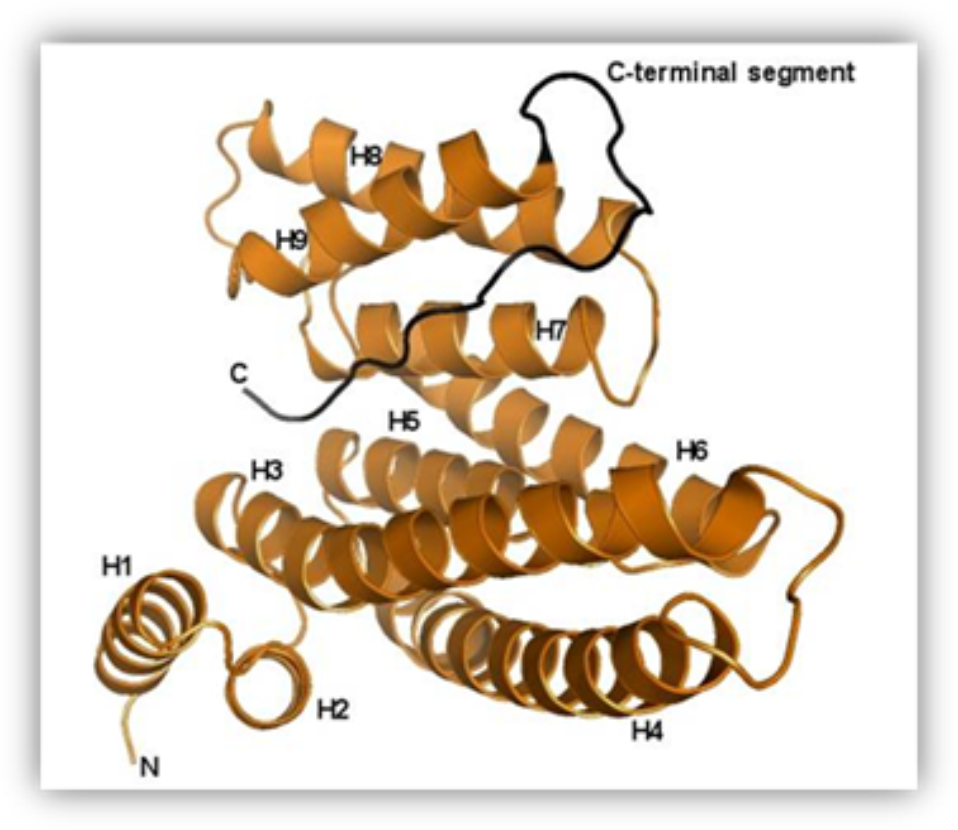
Structure of the human 14-3-3 protein (isoform zeta) with the modeled C-terminal segment.
Unraveling the mechanism of inhibition of human ubiquitin ligase Nedd4-2 by 14-3-3 protein
The neural precursor cell expressed developmentally downregulated 4 (Nedd4-2) is a member of the HECT E3 ubiquitin ligase family. As such, this enzyme targets proteins for ubiquitination in mammalian programmed cell death. Nedd4-2 has modular multidomain architecture, consisting of an N-terminal C2 domain, four WW domains, which contain two conserved tryptophan residues and a proline residue, and a C-terminal catalytic HECT domain. Nedd4-2 is also regulated by phosphorylation on three sites (Ser342, Thr367 and Ser448) recognized by 14-3-3 protein. SAXS-based structural analysis combined with chemical cross-linking and fluorescence spectroscopy provided the first glimpse into the 14-3-3-mediated inhibition of Nedd4-2, showing that 14-3-3 binding induces a structural rearrangement of Nedd4-2 by altering interactions between its structured domains (Pohl et. al 2021). Furthermore, the formation of the complex causes both steric hindrance of the WW3 and WW4 domains and a conformational change of the catalytic HECT domain (Joshi et al., 2022). Because WW domains presumably mediate Nedd4-2 binding to its substrates, such occlusions combined with conformational changes in the catalytic domain likely affect substrate ubiquitination, thus explaining the 14-3-3-mediated modulation of the ubiquitination of some Nedd4-2 substrates.
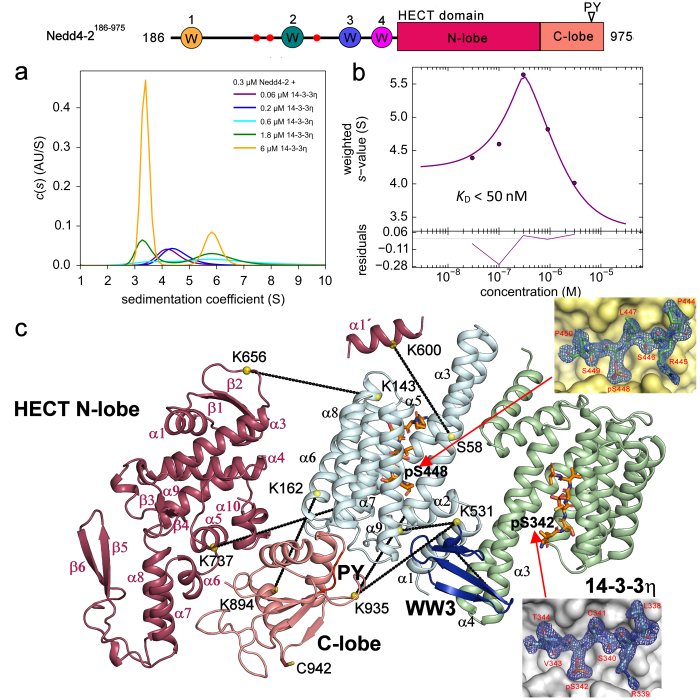
Structural analysis of the complex between Nedd4-2 and 14-3-3 protein by integrative structural biology approach. a)+b) Sedimentation velocity analytical ultracentrifugation (SV-AUC) measurements with the estimation of the apparent dissociation constant of the complex. c) SAXS-based structural modeling with the crystal structures of the 14-3-3 binding sites of Nedd4-2 (Pohl et. al (2021) Communications Biology).
14-3-3 proteins and ubiquitin ligase Nedd4-2
In 2021, using a series of biochemical and biophysical methods, we elucidated that the key sites for interaction with the 14-3-3 protein are the pSer342 and pSer448 sites, we elucidated the principle of this interaction by solving the crystal structure of 7NMZ, and we found that binding of the 14-3-3 protein blocks WW3 in the central channel of the 14-3-3 protein (Pohl et. al 2021).
In 2022, using fluorescence spectroscopy methods, we elucidated that the 14-3-3 protein reduces both mobility and solvent accessibility of the WW2, WW3 and WW4 domains of Nedd4-2. Conversely, binding of the 14-3-3 protien has the opposite effect on the active site of the HECT domain, which is more exposed to solvent and more mobile in the 14-3-3:Nedd4-2 complex (Joshi et al., 2022).
Current projects involve:
- The role of calcium ions in Nedd4-2 regulation and structure changes induced by calcium binding
- CryoEM of the 14-3-3:Nedd4-2 complex
- Searching for new E3 ubiquitin ligases regulated by 14-3-3 proteins
