Laboratory of Biological Rhythms

Content of this page
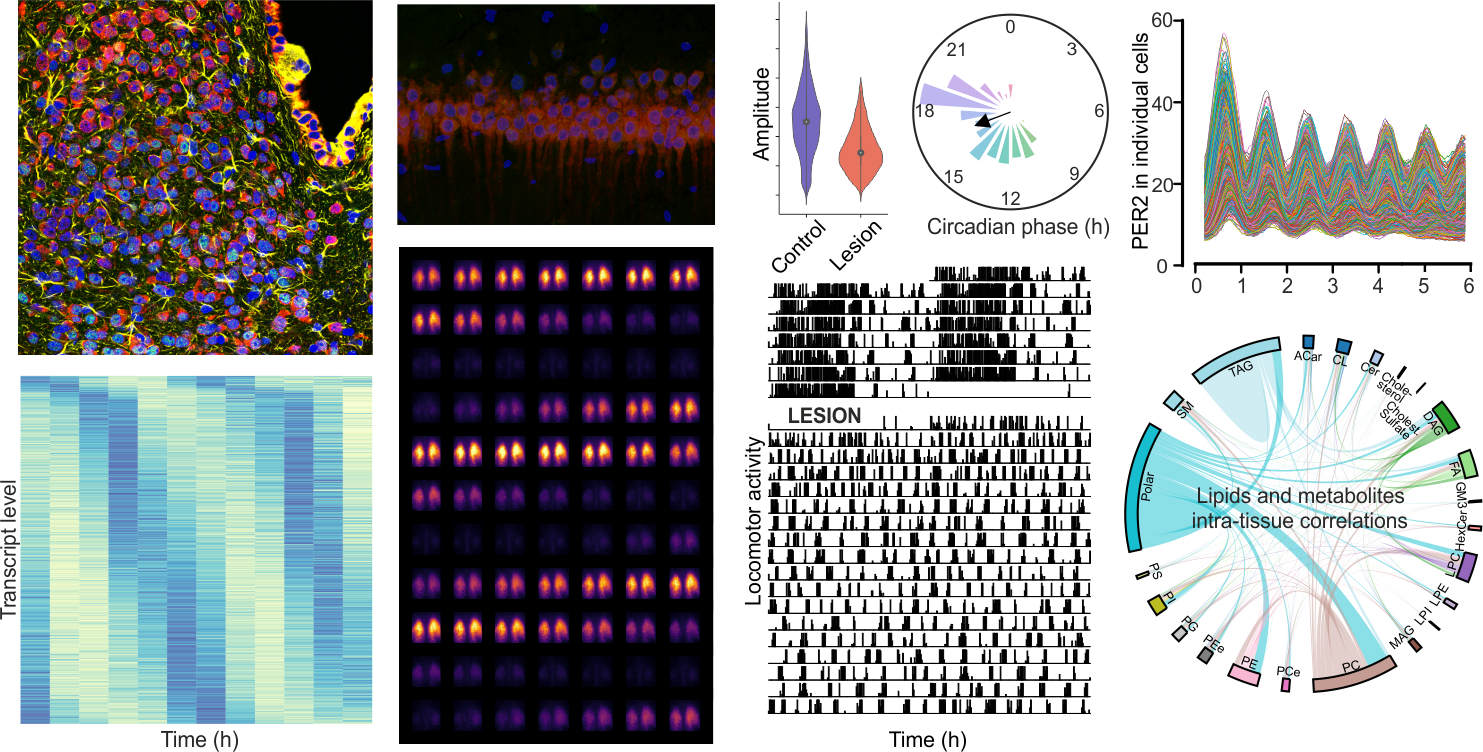
About the Laboratory
Please, consider filing out anonymous questionnaire regarding your chronotype and social jet lag.
GitHub
In the center of our interest is the endogenous time-keeping system, circadian clock of mammals, including humans. The system temporally regulates rhythmic processes in our body so that they take place at the proper time of day and are optimally synchronized relative to each other.
A failure of the temporal regulation has a negative impact on human health. Using in vivo and in vitro models, we study the molecular mechanisms underlying the circadian clock as well as the resulting physiological and behavioral rhythms.
We focus on the following questions:
- What are the mechanisms for entraining (synchronizing) the circadian system and what are the consequences of its failure?
- How does the circadian system develop and how is it being entrained during ontogeny?
- How is the circadian system of humans entrained in real life conditions, both in health and in disease?
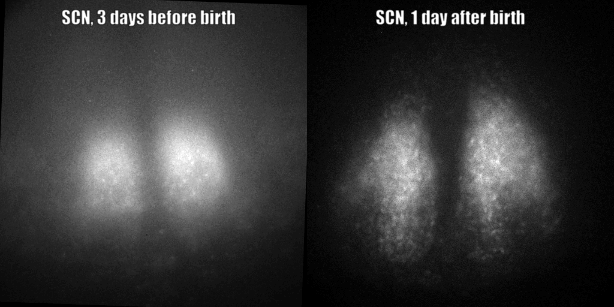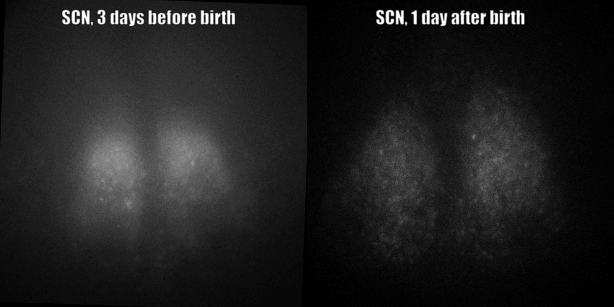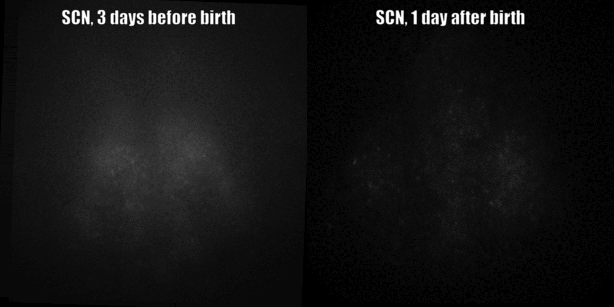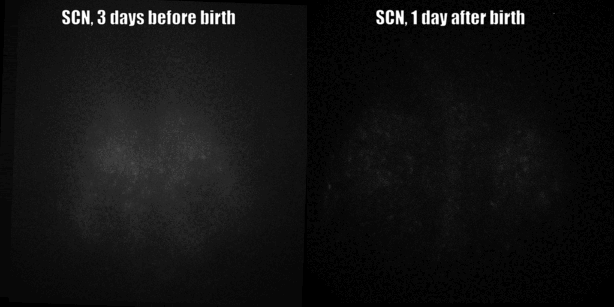
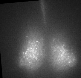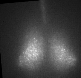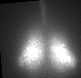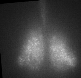
Fig. PER2 protein in the prenatal and early postnatal SCN, ex vivo luminescence microscopy.
Projects
Achievements
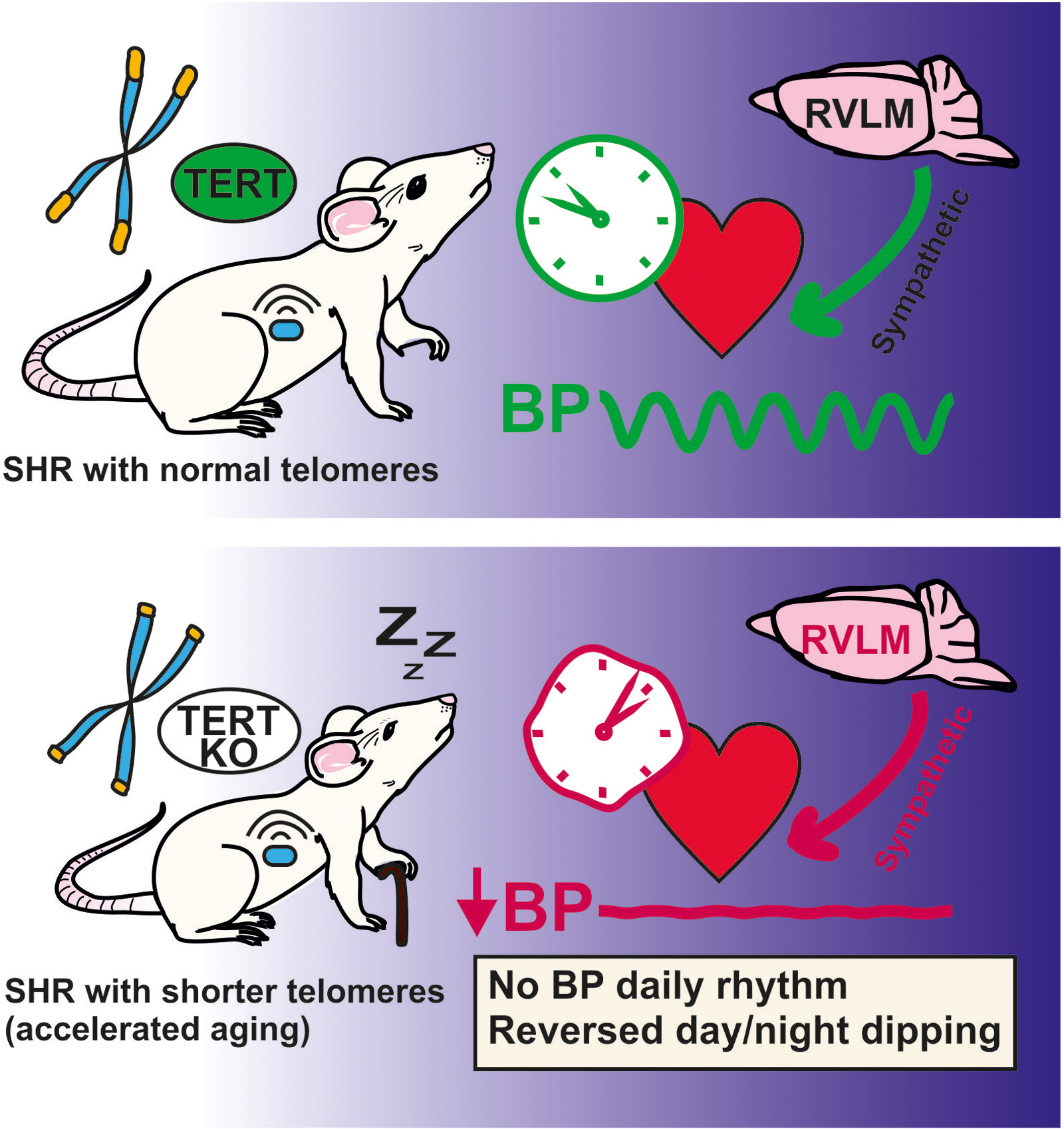
New publication (IF=8.2): Tert Deletion Impairs Circadian Regulation of Blood Pressure in Male Spontaneously Hypertensive Rats. Hypertension (2026)
Kateryna Semenovykh, Michal Pravenec, Ivana Vaněčková, Pavel Houdek, Martin Sládek, Miroslava Šimáková, Petr Mlejnek, Saba Selvi, Jan Šilhavý, František Liška, Dmytro Semenovykh, Silvie Hojná, Alena Sumová. Hypertension, 83(2): 579–590. doi: 10.1161/HYPERTENSIONAHA.125.25510
Telomere shortening is associated with the aging process. We have created a new genetic rat model with deletion of the Tert gene in which the F3 generation showed shortened telomeres in various tissues. We used spontaneously hypertensive rats, which develop hypertension and are closely associated with higher susceptibility to circadian dysregulation. The rats exhibited signs of accelerated aging at the behavioral level although the circadian regulation of locomotor activity was not impaired. In the rostral ventromedial medulla of the brainstem, the number of TH (tyrosine hydroxylase)-immunopositive cells was reduced, indicating a decrease in sympathetic tone. Importantly, the day/night rhythmicity of clock gene expression in the heart and other peripheral tissues was modulated in constant darkness, which was reflected in the complete abolition of the daily rhythm of heart rate and blood pressure. This is the first study to show the effects of deletion of Tert on peripheral circadian clocks and regulation of cardiovascular function at a systemic level.
New publication (IF=7.2): Maternal food-derived signals oscillate in the fetal suprachiasmatic nuclei before its circadian clock develops. PLoS Biology (2025)
Martin Sládek, Pavel Houdek, Tomáš Čajka, Alena Sumová. PLoS Biol 23(9): e3003404. doi: 10.1371/journal.pbio.3003404
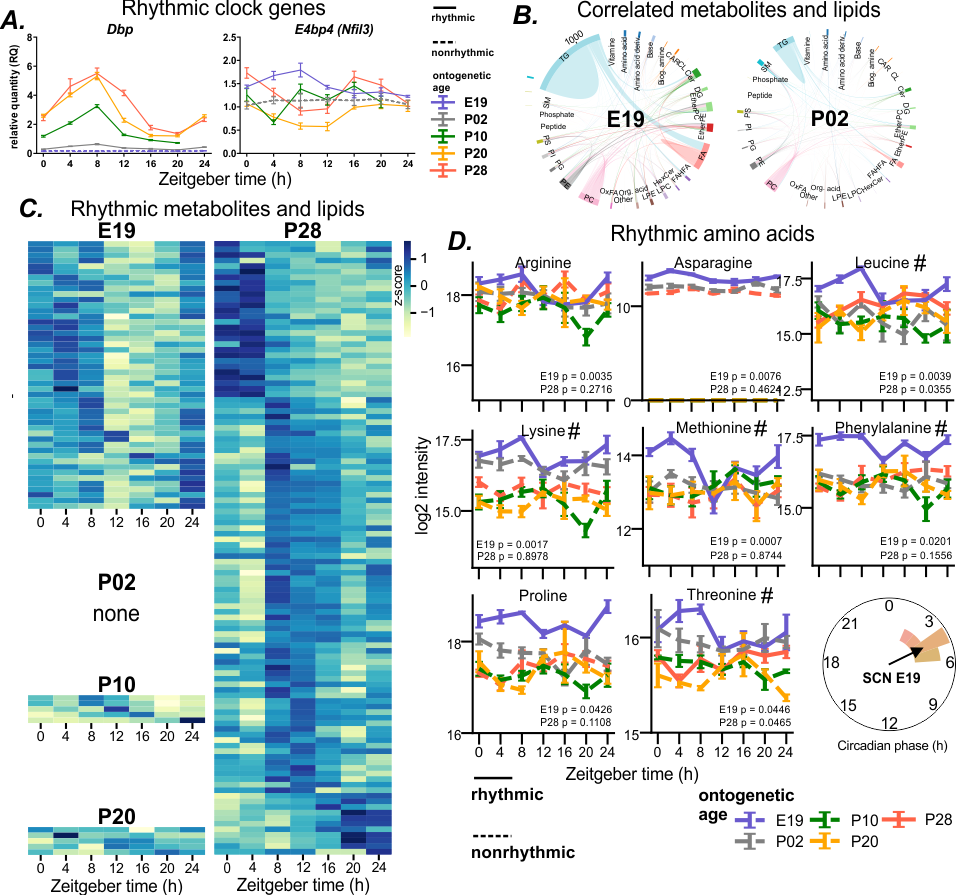
The development of the circadian clock in the suprachiasmatic nucleus (SCN) and its dependence on maternal cues remain poorly understood. We examined SCN ontogenesis in rats from late fetal to post-weaning stages, combining daily gene expression with time-resolved metabolomic and lipidomic profiling. Canonical clock gene rhythms (Per2, Nr1d1, Bmal1) were absent at E19 and gradually emerged by P10, while Dbp rhythmicity matured later (P20-28). Strikingly, before the SCN molecular clockwork established, metabolism-sensitive gene E4bp4 as well as essential amino acids and several other maternal food-derived metabolites displayed rhythmicity in the fetal SCN. The temporal coherence of the fetal SCN metabolome and lipidome declines markedly and its rhythmicity disappears immediately after birth, but reappears later in the postnatal period. Our findings uncover a previously unrecognized mechanism by which maternal metabolic signals provide temporal information to the fetal SCN before its circadian clock develops.
Fig. (A) Development of clock gene expression in the suprachiasmatic nucleus (SCN) analyzed by RT qPCR at various ontogenetic ages shows differences in their sensitivity to maternal rhythmic cues. (B) Amount of correlations between metabolites and lipids decrease after birth. (C) Number of rhythmic compounds decreases after birth and dramatically increases after weaning. (D) Essential amino acids are rhythmic with a similar phase in the SCN before birth.
New publication (IF=7.7): Feeding regime synchronizes circadian clock in choroid plexus - insight into a complex mechanism. Cell Mol Life Sci (2025)
Tereza Dočkal, Pavel Houdek, Kateryna Semenovykh, Revan Rangotis, Martin Sládek, Alena Sumová. Feeding regime synchronizes circadian clock in choroid plexus – insight into a complex mechanism. Cell Mol Life Sci. 2025 Jun 23;82(1):247. doi: 10.1007/s00018-025-05798-3
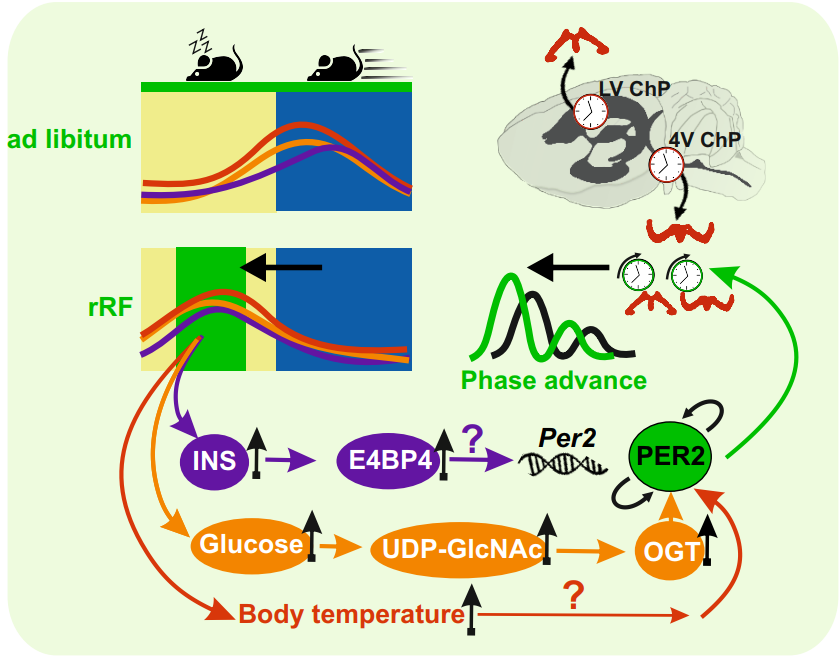
The circadian clock in the choroid plexus (CP) controls a number of physiological processes important for its function, but the signals that synchronize this clock have not yet been thoroughly investigated. In our study, we found that the clock in the ChP in the fourth ventricle (4V) is more robust than the clock in the lateral ventricle (LV). We were therefore interested in whether this clock uses the time of food intake as a signal to synchronize, and whether this signal has a different effect in the ChP in 4V and LV.
To understand this mechanism, we exposed mPer2Luc mice for ten days to a regimen during which food was present for only 6 hours, during the light (resting) phase. This resulted in a shift in the clock phase in both the 4V and LV ChP. We confirmed this by monitoring the rhythm of bioluminescence in isolated ChP tissues as well as changes in diurnal clock gene expression directly in mice. In contrast, clocks in other brain regions (e.g. DMH, ARC, LHb) were not shifted by this regime. That is, the biological clock in the choroid plexus synchronizes with the new regime in food intake, while the clocks in other parts of the brain remain set by light. ChP in 4V responded more strongly to the changed feeding time than ChP in LV.
The mechanism involves a food-induced increase in insulin, glucose and body temperature levels that directly affect the molecular clock. The effect of glucose was partially blocked by OSMI-1, suggesting that a protein modification called O-GlcNAc may play a role in the process.
It turns out that the choroid plexus is not only sensitive to signals from the light-driven central clock, but can also rapidly readjust its rhythm according to the timing of food intake.
Fig. Restricting food intake to 6 hours during the day is associated with a shift in the phase of the clock in the choroid plexus. The mechanism of the shift involves food-induced increases in insulin, glucose and body temperature, which directly affect the molecular clock mechanism.
New publication (IF=6.4): Chronodisruption that dampens output of the central clock abolishes rhythms in metabolome profiles and elevates acylcarnitine levels in the liver of female rats. Acta Physiologica (2025)
Shiyana Arora, Pavel Houdek, Tomáš Čajka, Tereza Dočkal, Martin Sládek, Alena Sumová. Acta Physiologica. 2025 Jan 13. doi: 10.1111/apha.14278
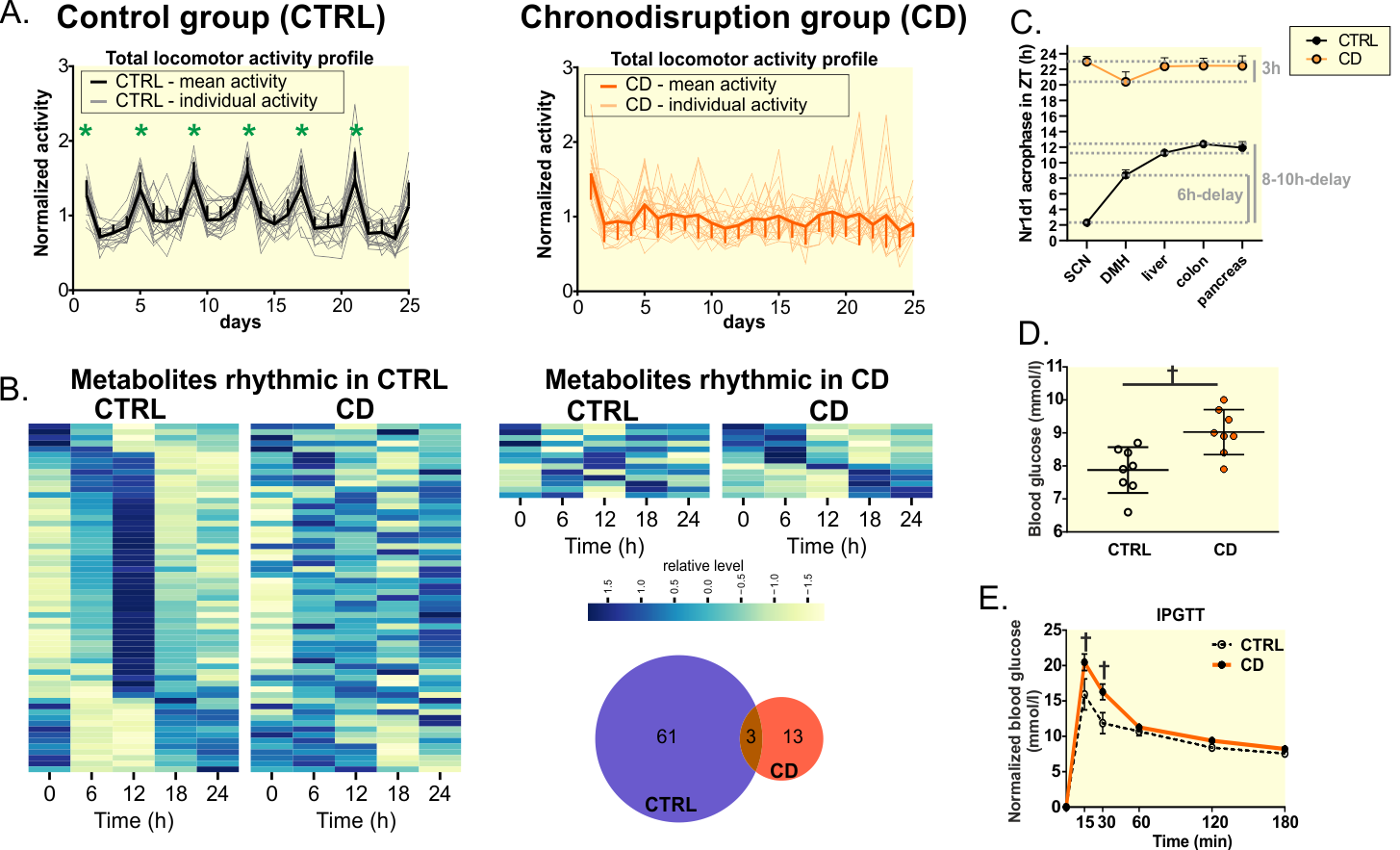
Exposure to light at night and meal time misaligned with the light/dark (LD) cycle – typical features of daily life in modern 24/7 society – are associated with negative effects on health. To understand the mechanism, we developed a novel protocol of complex chronodisruption (CD) in which we exposed female rats to four weekly cycles consisting of 5-day intervals of constant light and 2-day intervals of food access restricted to the light phase of the 12:12 LD cycle. We showed that CD attenuated the rhythmic output of the central clock in the suprachiasmatic nucleus via Prok2 signaling, thereby disrupting locomotor activity, the estrous cycle, sleep patterns, and mutual phase relationship between the central and peripheral clocks. In the periphery, CD abolished Per1,2 expression rhythms in peripheral tissues and worsened glucose homeostasis. In the liver specifically, it impaired the expression of NAD+, lipid and cholesterol metabolism genes and abolished most of the high-amplitude rhythms of lipids and polar metabolites. Interestingly, CD abolished the circadian rhythm of Cpt1a expression and increased the levels of long-chain acylcarnitines (ACar 18:2, ACar 16:0), indicating enhanced fatty acid oxidation in mitochondria. Our data show the widespread effects of CD on metabolism and point to ACars as biomarkers for CD due to misaligned sleep and feeding patterns.
Fig. A. Representative mean activity profiles of female rats from CTRL (left) or chronodisrupted (CD, right) groups with green rectangles and green stars depicting increased activity during estrus phase. B. Heatmap and Venn diagram of lipids and polar metabolites identified as significantly rhythmic in CTRL (left) or CD (right) liver samples. C. Circadian phase of Rev-Erbα mRNA in the suprachiasmatic nucleus (SCN), dorsomedial hypothalamus (DMH) and peripheral tissues from CTRL (black) and CD (orange) group calculated by cosinor. Notice the effect of CD on phase relationship between the SCN and periphery. D. Blood glucose was significantly higher (t-test, P = 0.0047) in CD group. E. Intraperitoneal glucose tolerance test (IPGTT) showed significantly slower clearance of injected glucose load (elevated levels 15 and 30 min after the glucose injection, 2-way ANOVA with Sidak’s test, P = 0.0055 and P = 0.0075, respectively).
New publication (IF=7.3): The circadian clock in the choroid plexus drives rhythms in multiple cellular processes under the control of the suprachiasmatic nucleus. Fluids and Barriers of the CNS (2024)
Martin Sládek, Pavel Houdek, Jihwan Myung, Kateryna Semenovykh, Tereza Dočkal, Alena Sumová. Fluids Barriers CNS. 2024 May 27;21(1):46. doi: 10.1186/s12987-024-00547-3.
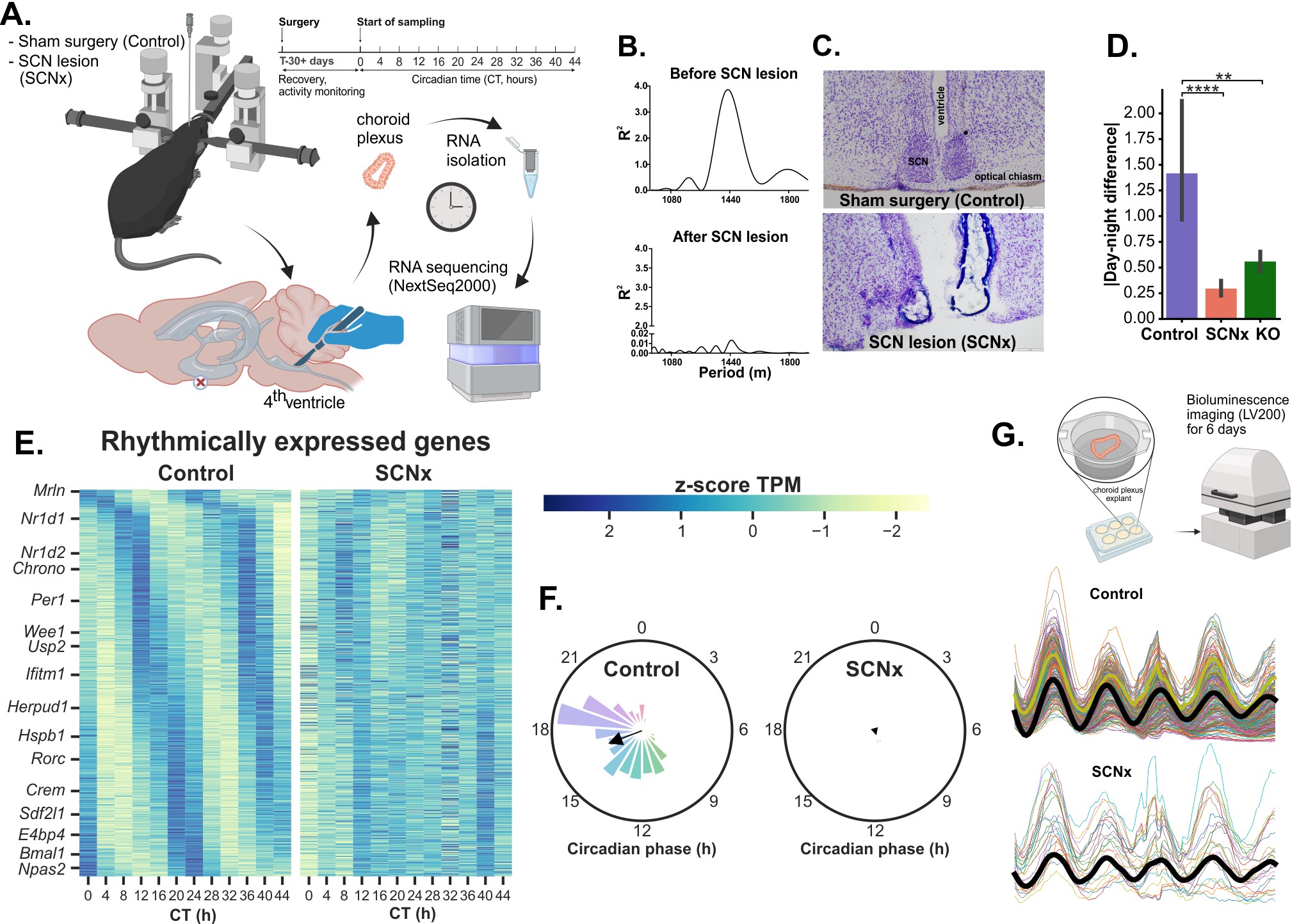
Choroid plexus (ChP), the brain structure primarily responsible for cerebrospinal fluid production, contains a robust circadian clock with unknown function. We used genetic mouse models (WT, mPer2Luc, ChP-specific Bmal1 knockout) combined with surgical lesion of the SCN (SCNx), time-resolved transcriptomics, and single cell luminescence microscopy. We found that the ChP clock regulates cerebrospinal fluid circadian secretome, precisely times endoplasmic reticulum stress response, and controls genes involved in neurodegenerative diseases. In ChP of SCNx mice, the rhythms were severely dampened to a comparable extent as in mice with ChP-specific Bmal1 knockout; they were restored by daily injections of dexamethasone. Our data demonstrate that the ChP clock controls tissue-specific gene expression and is strongly dependent on the presence of a functional connection with the SCN. The results may contribute to the search for a novel link between ChP clock disruption and impaired brain health.
Fig: A. Workflow. B. Period of locomotor activity in Control and SCNx mice. C. SCN after sham surgery and after lesion. D. Rhythms in SCNx ChP are dampened to similar extent as rhythms in ChP from tissues-specific Bmal1-KO. E. Rhythmic genes in Control (left) or SCNx (right) ChP samples. F. Phase histogram of genes Control (left) or SCNx (right) ChP samples. G. PER2::LUCIFERASE rhythms in single-cell sized ROIs across an explanted ChP from either Control or SCNx mice.
New publication (IF=15): Circadian clock in choroid plexus is resistant to immune challenge but dampens in response to chronodisruption. Brain Behav Immun (2024)
Milica Drapšin, Tereza Dočkal, Pavel Houdek, Martin Sládek, Kateryna Semenovykh, Alena Sumová. Brain Behav Immun. 2024 Jan 25:S0889-1591(24)00229-0. doi: 10.1016/j.bbi.2024.01.217.
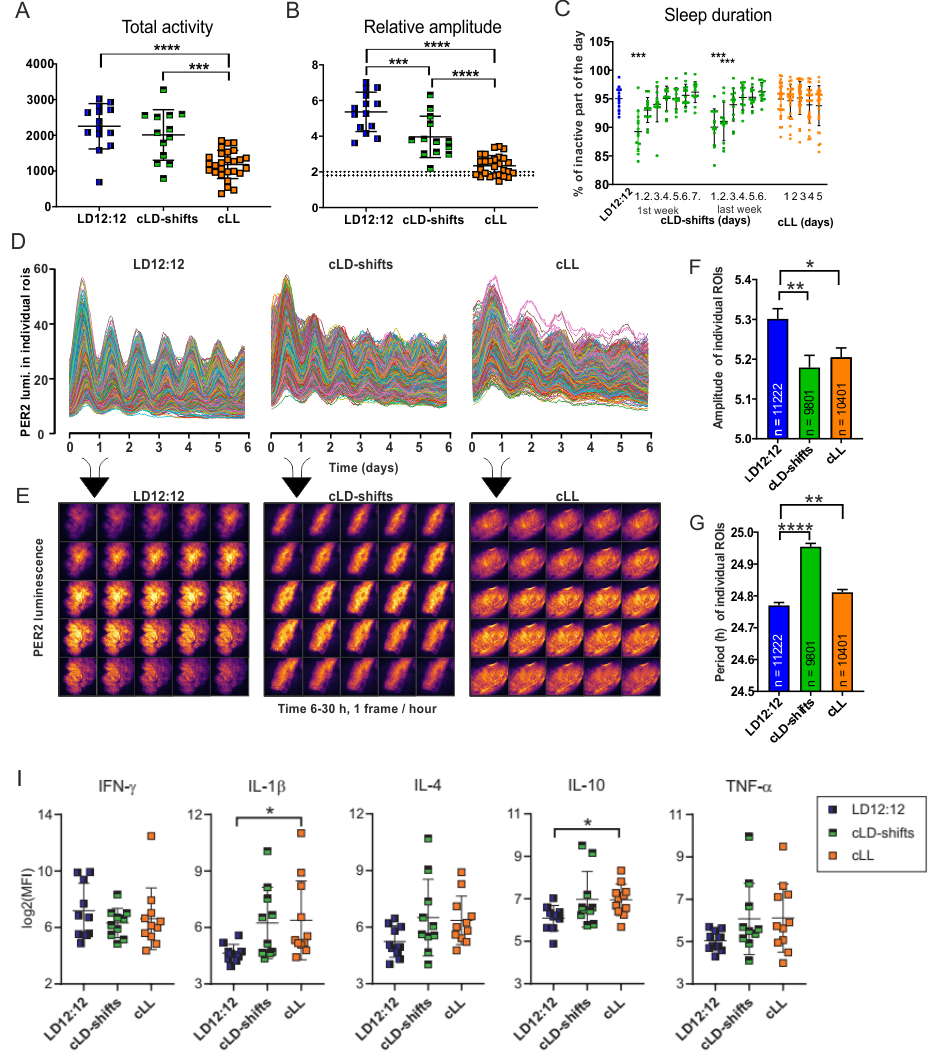
Choroid plexus (ChP) in brain ventricles plays significant role in brain homeostasis. ChP cells contain robust circadian oscillators that control its function. Chronodisruption in the form of exposure to constant light (cLL) and chronic advance of the light-dark cycle (cLD-shifts) impaired the ChP clock due to disrupted signals originating from the SCN involving glucocorticoid signaling. Sleep patterns and immune markers in the brain were also affected. Interestingly, LPS systemic inflammation did not influence the ChP clock, but disrupted the liver clock. The data highlight the role of ChP in protecting the brain from neuroinflammation. Our results provide a novel link between human lifestyle-induced chronodisruption and the impairment of ChP-dependent brain homeostasis.
Fig. Choroid plexus (ChP) chronodisruption. (A) Total locomotor activity, (B) relative amplitude, and (C) sleep duration of mice reared on
normal light-dark regime (LD12:12), on a chronic phase-shifted light-dark (cLD-shifts) cycle regime, or on a constant light (cLL) regime.
(D-E) 6-day luminescence microscopy of the circadian expression of PER2 protein imaged in ChP explants of analyzed mice,
quantitative analysis of amplitude (F) and period (G) of individual ChP cells. (I) Multiplex analysis of cytokines in plasma of analyzed mice.
New pulblication (IF=6.3): Population-representative study reveals cardiovascular and metabolic disease biomarkers associated with misaligned sleep schedules. Sleep (2023)
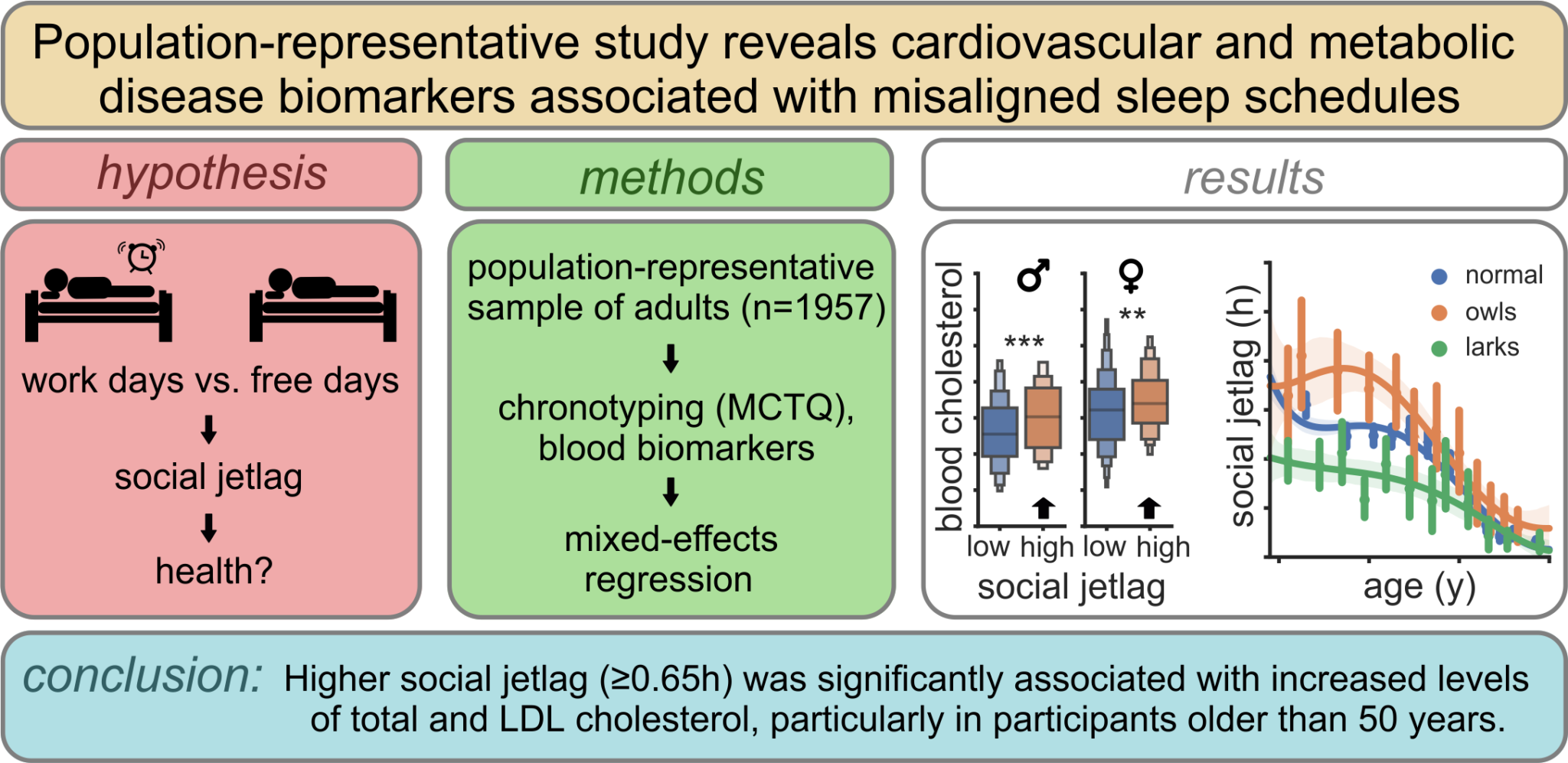
Martin Sládek, Jan Klusáček, Dana Hamplová and Alena Sumová. Sleep 2023 Feb 24, 10.1093/sleep/zsad037. Social jetlag, or chronic misalignment between biological and social time, is the most common disturbance of circadian rhythms in modern society.
It remains unclear, however, whether social jetlag has any negative health effects. By investigating a unique population-representative dataset, including 1957 blood samples analyzed for nine biomarkers, we report significant associations between sleep phase preference, social jetlag and cardio-metabolic biomarkers. We identify novel factors affecting social jetlag and demonstrate that flexible working schedule efficiently alleviates social jetlag.
New publication (IF=7.4): Lithium affects the circadian clock in the choroid plexus – A new role for an old mechanism. Biomedicine & Pharmacotherapy (2023)
Karolína Liška , Tereza Dočkal , Pavel Houdek , Martin Sládek, Vendula Lužná , Kateryna Semenovykh , Milica Drapšin and Alena Sumová.
Lithium affects the circadian clock in the choroid plexus – A new role for an old mechanism. Biomedicine & Pharmacotherapy 159 (2023), 114292
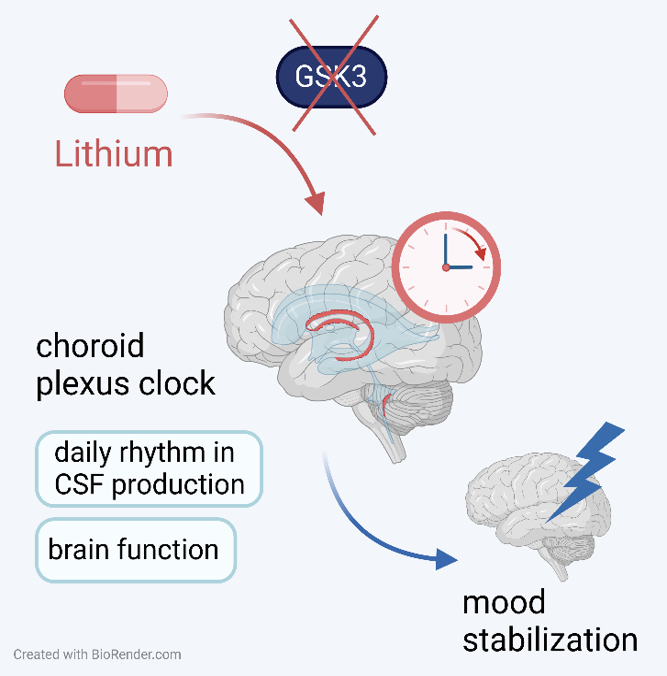
Lithium is an effective mood stabilizer used in the treatment of Bipolar Disorder (BD), but the mechanism of its therapeutic action is not well understood. We investigated the effect of lithium on the circadian clock located in the ventricle barrier complex containing the choroid plexus (CP), a part of the glymphatic system that influences gross brain function via the production of cerebrospinal fluid. Using in vivo and ex vivo methods, analyzing clock gene expression and probing cell-signaling pathways of the CP, we demonstrated that lithium strongly affected the circadian clock in the CP. The effect of lithium was not mediated by GSK3b-exclusive mechanism, but rather by modulation of PKC activity. The effects on the CP clock may be involved in the therapeutic effects of lithium and improve brain function in BD patients by aligning the function of the CP clock-related glymphatic system with the sleep-wake cycle. Importantly, our data argue for personalized timing of lithium treatment in BD patients.
Fig: Lithium affects the circadian clock in the choroid plexus independently of GSK3, resulting in time-dependent phase shifts that may affect CSF production, brain function and its therapeutic outcome.
New publication (IF=9.6): Early rhythmicity in the fetal suprachiasmatic nuclei in response to maternal signals detected by omics approach. Greiner P, Houdek P, Sládek M, Sumová A (2022) PLoS Biol 20(5): e3001637.
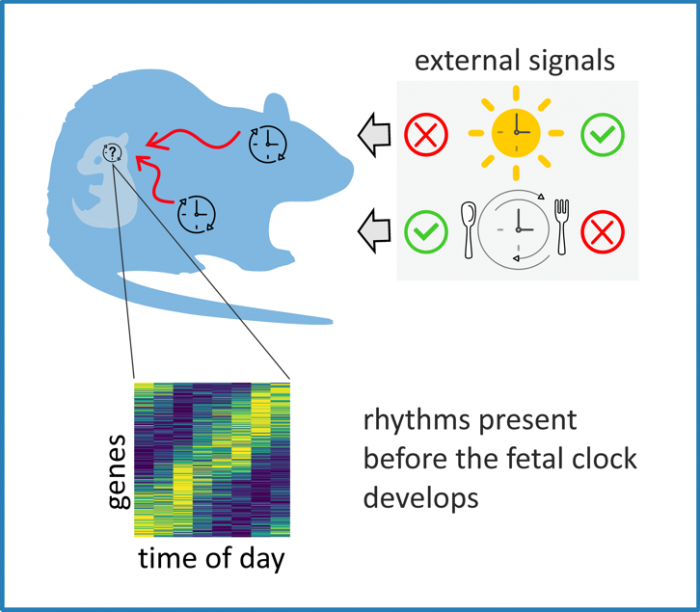
Fig. Maternal signals drive rhythmicity in the fetal suprachiasmatic nuclei before their molecular clock develops.
doi:10.1371/journal.pbio.3001637.g005. Our results indicate the importance of a well-functioning maternal biological clock in providing rhythmic environment during the fetal brain development.
During development in of the fetal suprachiasmatic nuclei, maternal stimuli may substitute for an absent inter-cellular web of synapses and drive cell-population rhythms before the SCN clock fully matures. Distinct maternal signals rhythmically control a variety of neuronal processes in the fetal rat suprachiasmatic nuclei before they begin to operate as the central circadian clock.
New publication (IF=9.3): Misaligned feeding schedule elicits divergent circadian reorganizations in endo- and exocrine pancreas clocks. Honzlová P, Z Novosadová, P Houdek, M Sládek and A Sumová. Cell Mol Life Sci 2022 May 27;79(6):318
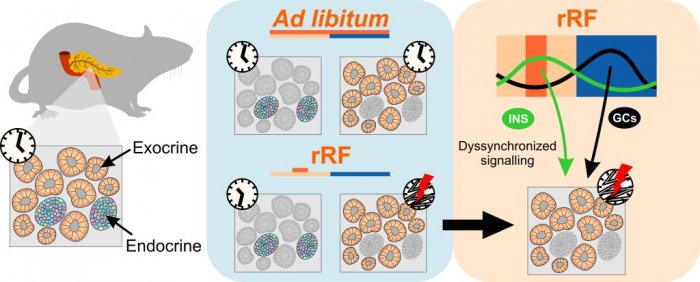
doi:10.1007/s00018-022-04354-7. Misaligned meals become a frequent lifestyle transgression related to current societal pressure imposed on behavior.
New publication: Modulation of single cell circadian response to NMDA by diacylglycerol lipase inhibition reveals a role of endocannabinoids in light entrainment of the suprachiasmatic nucleus. Sladek, Liska, Houdek, Sumová. Neuropharmacology 185 (2021)
doi:10.1016/j.neuropharm.2021.108455. The paper provides new insights into the modulatory role of endocannabinoid signaling during the light entrainment of the SCN.
Suprachiasmatic nucleus (SCN) of the hypothalamus is the master clock that drives circadian rhythms in physiology and behavior and adjusts their timing to external cues. Neurotransmitter glutamate and glutamatergic receptors sensitive to N-methyl-D-aspartate (NMDA) play a dual role in the SCN by coupling astrocytic and neuronal single cell oscillators and by resetting their phase in response to light. Recent reports suggested that signaling by endogenous cannabinoids (ECs) participates in both of these functions. ECs, such as 2-arachidonoylglycerol (2-AG), act via presynaptic CB1 receptors to modulate synaptic activity and affect the SCN response to light-mimicking NMDA stimulus in a time-dependent manner. We demonstrate that circadian clock in the rat SCN regulates expression of 2-AG transport, synthesis and degradation enzymes as well as its receptors. Inhibition of the major 2-AG synthesis enzyme, diacylglycerol lipase, enhances the phase delay and lowers the amplitude of explanted SCN rhythm in response to NMDAR activation. Using PER2 bioluminescence imaging, we show how single cell oscillators reflect the phase response of the whole SCN. Additionally, we present strong evidence that the zero amplitude behavior of the SCN in response to single NMDA stimulus in the middle of subjective night is the result of a loss of rhythm in individual SCN cells. The paper provides new insights into the modulatory role of endocannabinoid signaling during the light entrainment of the SCN.

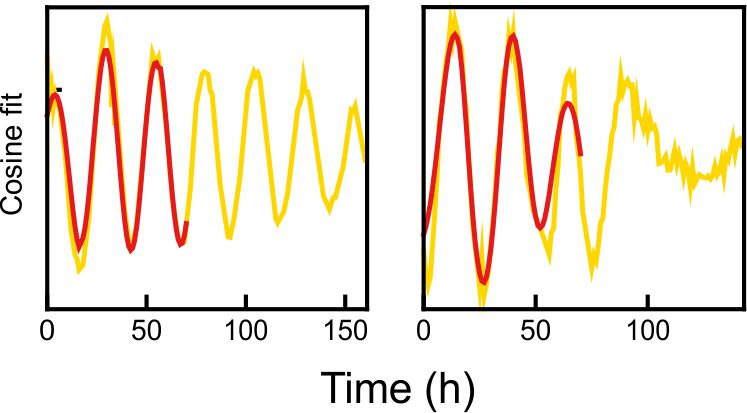
Fig 1. Explanted SCN from mPer2Luc mouse oscillating in vitro was treated by diacylglycerol lipase inhibitor and NMDA during daytime (left) or in the middle of subjective nighttime (right).
Fig 2. PER2 levels in a single representative neuron from the explants shown in Fig. 2. Red line shows the signal before treatment.
Publications
Marhefková; N. - Sládek; Martin - Klusáček; Jan - Röschová; Michaela - Kahle; M. - Roland; R. - Pazderník; M. - Wohlfahrt; P. - Novodvorský; P. - Haluzík; M. - Sumová; Alena - Dubský; M. Effects of type 1 diabetes on sleep duration and timing – a comparison within the general population. Sleep Medicine. 2025; 136(December); 106868.
IF = 3.4
Rudl Kulhavá; Lucie - Houdek; Pavel - Nováková; Michaela - Hricko; Jiří - Paučová; Michaela - Kuda; Ondřej - Sládek; Martin - Fiehn; O. - Sumová; Alena - Čajka; Tomáš Circadian ontogenetic metabolomics atlas: an interactive resource with insights from rat plasma; tissues; and feces. Cellular and Molecular Life Sciences. 2025; 82(28 Jun); 264.
IF = 6.2
Arora; Shiyana - Houdek; Pavel - Čajka; Tomáš - Dočkal; Tereza - Sládek; Martin - Sumová; Alena Chronodisruption that dampens output of the central clock abolishes rhythms in metabolome profiles and elevates acylcarnitine levels in the liver of female rats. Acta Physiologica. 2025; 241(2); e14278.
IF = 5.6