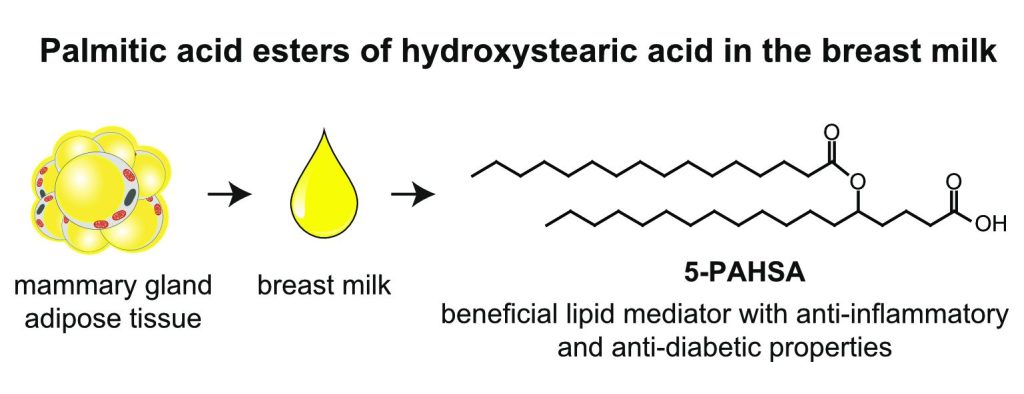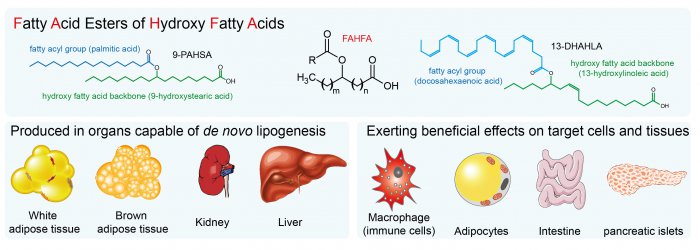
White adipose tissue (WAT) is a complex endocrine organ and its low-grade inflammation in obesity contributes to the development of metabolic disorders. In 2014, a class of WAT-born lipid mediators – fatty acid esters of hydroxy fatty acids (FAHFA) was discovered [1]. FAHFAs are endogenous lipids with anti-inflammatory and anti-diabetic properties, including the enhancement of glucose tolerance, and insulin and glucagon-like peptide 1 (GLP-1) secretion while reducing inflammatory responses [1-5]. They consist of a fatty acid (e.g. palmitic acid, PA) esterified to the hydroxyl group of a hydroxy fatty acid (e.g. hydroxystearic acid, HSA), abbreviated as PAHSA. The position of the branching carbon defines a regioisomer (e.g. 5-PAHSA). There are several regioisomer families derived from palmitic, palmitoleic, stearic, oleic, linoleic, and docosahexaenoic acid with tissue-specific distribution documented so far [1-4, 6, 7]. Adipose tissue represents a major site of FAHFAs synthesis [1, 2], but the biosynthetic enzymes involved are unknown [11]. Serine hydrolase carboxyl ester lipase [8] and threonine hydrolases [9] were identified as FAHFA-metabolizing enzymes. In humans, FAHFAs were detected in the serum, breast milk, meconium, and adipose tissues [1, 2, 10].
Example of PAHSA regioisomers:
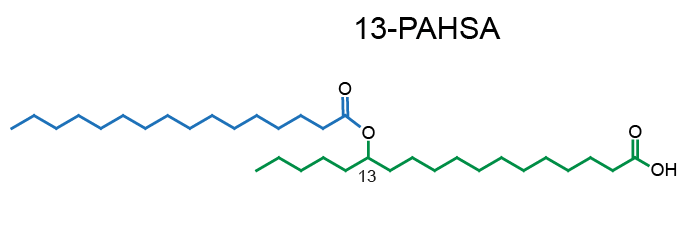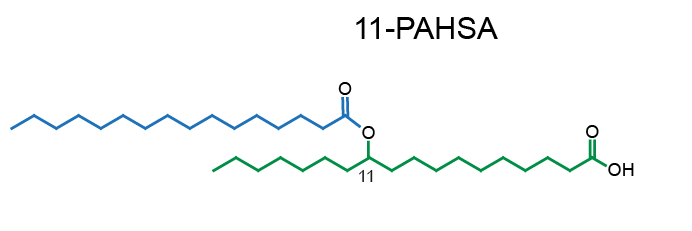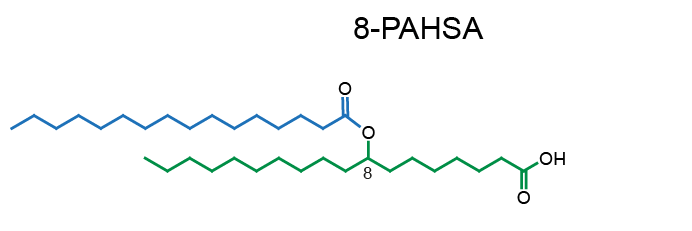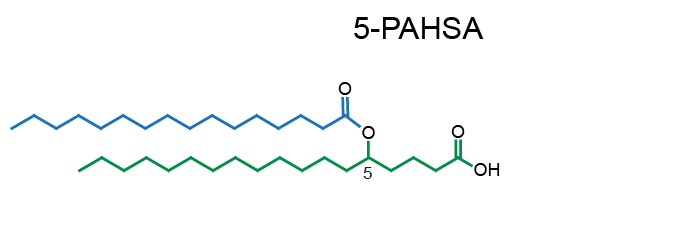
Network representation of FAHFA families linked according to the hydroxy-backbone and colored according to the esterified fatty acid [11] (click for full image).

Our hypothesis is that novel FAHFAs derived from omega-3 PUFA, with anti-inflammatory properties, could be found in mice and humans and that they can beneficially affect adipose tissue metabolism in obesity, especially low-grade inflammation. We are also interested in FAHFA metabolic pathways, which seem to be as complex as eicosanoid-related pathways. Using experiments in cell cultures, mice and humans we explore the structures, effects on WAT inflammation, WAT glucose tolerance and molecular mechanisms of signaling of these new lipokines. Our results present a significant advance in research of the mechanisms connecting inflammation, metabolism, and nutritional lipids.
Metabolomics lab team members:
- Ondrej Kuda (PI)
- Marie Brezinova (PhD student)
- Martina Rombaldova (PhD student)
- Marina Oseeva (PhD student)
- Daniela Salkova (technician)
Grants
Supported by:
- Czech Science Foundation project no. 17-10088Y (2017-2019, PI: Ondrej Kuda)
- MEYS project no. LTAUSA17173 (2017-2019, PI: Ondrej Kuda)
- MEYS project no. LH14040 (2014-2016, PI: Ondrej Kuda)
Our publications:
► Ondrej Kuda✉ On the Complexity of PAHSA Research. Cell Metabolism 2018, Sep 20; DOI https://doi.org/10.1016/j.cmet.2018.09.006
Comments on the methodological and conceptual problems when working with FAHFAs.
fulltext at https://www.cell.com/cell-metabolism/pdf/S1550-4131(18)30571-0.pdf
free fulltext link https://authors.elsevier.com/a/1Xq8i5WXUlA-Mk
► Ondrej Kuda✉, Marie Brezinova, Jan Silhavy, Vladimir Landa, Vaclav Zidek, Chandra Dodia, Franziska Kreuchwig, Marek Vrbacky, Laurence Balas, Thierry Durand, Norbert Hübner, Aron B. Fisher, Jan Kopecky and Michal Pravenec Nrf2-mediated Antioxidant Defense and Peroxiredoxin 6 are Linked to Biosynthesis of Palmitic Acid Ester of 9-Hydroxystearic Acid. Diabetes 2018 Mar; db171087.; DOI https://doi.org/10.2337/db17-1087
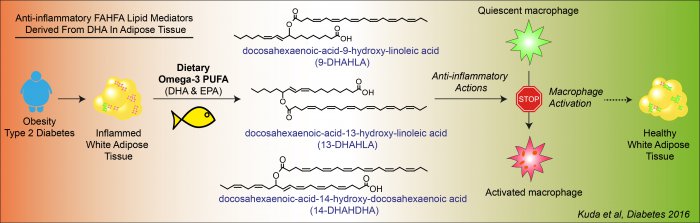
Omega-3 polyunsaturated fatty acids (omega-3) of marine origin alleviate inflammation, while having favorable metabolic effects. Omega-3 reduce the risk of development of cardiovascular disorders that are linked to obesity and type 2 diabetes, and also improve lipid metabolism. A complex research of omega-3-related mechanisms of action in mouse models of obesity at the Institute of Physiology CAS, clinical research on obese patients with type 2 diabetes in the Institute for Clinical and Experimental Medicine, and a collaboration with the Institute of Organic Chemistry and Biochemistry CAS led to the identification of structures of novel signaling molecules of lipid origin – esters of fatty acids and hydroxyl-fatty acids (FAHFA) – derived from docosahexaenoic acid (DHA): 13-DHAHLA, 9-DHAHLA a 14-DHAHDHA. These molecules, which are synthesized by adipose cells and exert anti-inflammatory effects, were detected in the serum and adipose tissue of both obese mice and diabetic patients following dietary intervention with omega-3. These newly discovered molecules, which can be endogenously synthesized when eating an appropriate diet, are involved in the beneficial health effects of omega-3 and have the potential for their wide use in the prevention and treatment of severe diseases.
Chronic low-grade inflammation contributes to the development of diabetes, as well as cardiovascular, gastrointestinal and certain brain disorders. Lipids of marine origin help to prevent inflammatory diseases.
http://diabetes.diabetesjournals.org/content/65/9/2580
http://diabetes.diabetesjournals.org/content/65/11/3516.2 erratum – an incorrect version of the Supplementary Data was erroneously posted online and has been replaced with the correct version.
Purification of 13-DHAHLA: Organic synthesis of docosahexaenoic acid-13-hydroxylinoleic acid (13-DHAHLA) was performed according to Steglich esterification from docosahexaenoic acid (DHA) and 13-hydroxylinoleic acid (13-HODE). The product was purified using silica-based Ag+ flash chromatography (low pressure silver-ion chromatography, Discovery Ag-Ion SPE sorbent, Sigma-Aldrich) and a combination of acetonitrile and acetone [12]).
Kuda O. Bioactive metabolites of docosahexaenoic acid. Biochimie. Jan 2017, DOI: 10.1016/j.biochi.2017.01.002
http://www.sciencedirect.com/science/article/pii/S0300908416302218
An integrative overview of how DHA is metabolized emphasizing the derivatives that have been identified as bioactive.
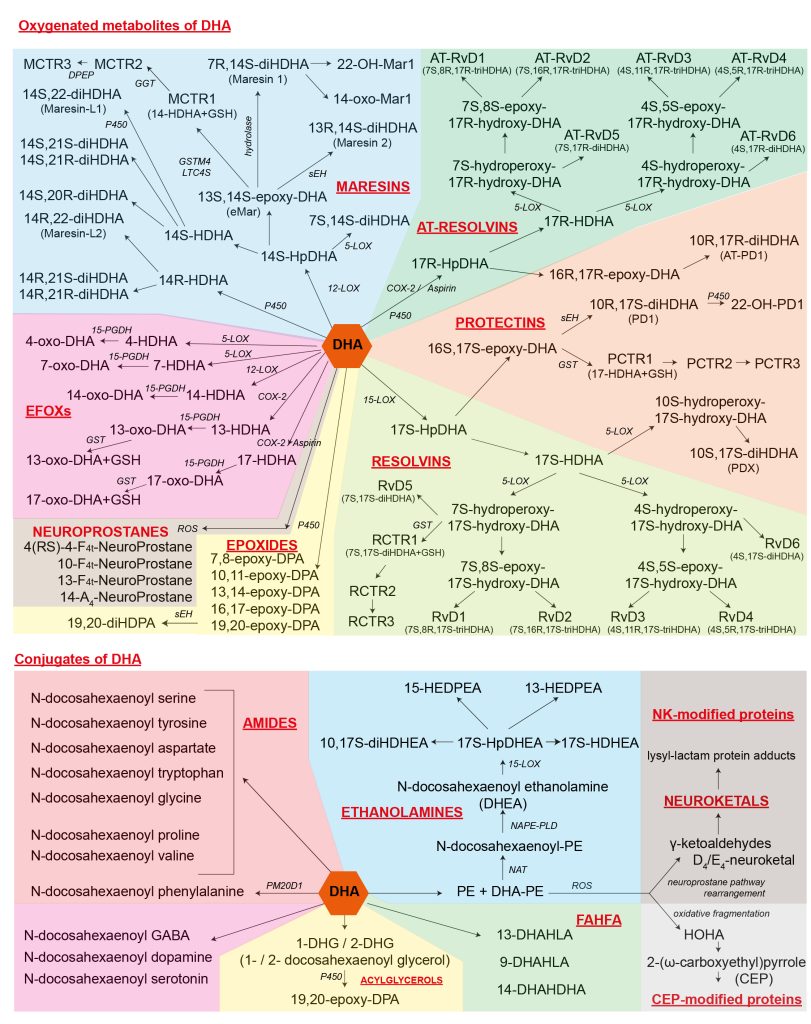 |
Legend: 13-DHAHLA, 13-(docosahexaenoyloxy)-hydroxylinoleic acid 14-DHAHDHA, 14-(docosahexaenoyloxy)-hydroxydocosahexaenoic acid 9-DHAHLA, 9-(docosahexaenoyloxy)-hydroxylinoleic acid AT-, aspirin-triggered- CEP, 2-(ω-carboxyethyl)pyrrole COX, cyclooxygenase DHEA, docosahexaenoyl ethanolamine DHG, docosahexaenoyl glycerol diHDHA, dihydroxydocosahexaenoic acid diHDHEA, dihydroxy-DHEA diHDPA, dihydroxydocosapentaenoic acid DPA, docosapentaenoic acid DPEP, dipeptidase eMar, 13,14-epoxy-maresin GGT, γ-glutamyl transferase GSH, glutathione GST, glutathione S-transferase GSTM4, glutathione S-transferase HEDPEA, hydroxy-epoxy-docosapentaenoyl ethanolamine HOHA, 4-hydroxy-7-oxohept-5-enoic acid HpDHA, hydroperoxydocosahexaenoic acid HpDHEA, hydroperoxy-DHEA LOX, lipoxygenase MCTR, Maresin conjugates in tissue regeneration NAPE-PLD, N-acyl phosphatidylethanolamine-specific phospholipase D NAT, N-acyltransferase P450, cytochrome P450 PCTR, Protectin conjugates in tissue regeneration PD, protectin D PE, phosphatidylethanolamine PGDH, hydroxyprostaglandin dehydrogenase RCTR, Resolvin conjugates in tissue regeneration ROS, reactive oxygen species RvD, resolvin D sEH, soluble epoxide hydrolase triHDHA, trihydroxydocosahexaenoic acid |
fulltext share link till 01/08/2018
Ondrej Kuda✉, Marie Brezinova, Jan Silhavy, Vladimir Landa, Vaclav Zidek, Chandra Dodia, Franziska Kreuchwig, Marek Vrbacky, Laurence Balas, Thierry Durand, Norbert Hübner, Aron B. Fisher, Jan Kopecky and Michal Pravenec Nrf2-mediated Antioxidant Defense and Peroxiredoxin 6 are Linked to Biosynthesis of Palmitic Acid Ester of 9-Hydroxystearic Acid. Diabetes 2018 Mar; db171087.; DOI https://doi.org/10.2337/db17-1087
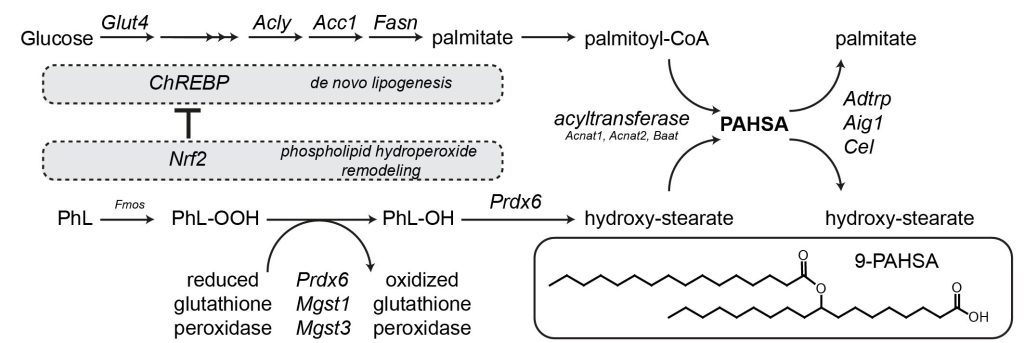
Comprehensive lipidomic analysis of rat white adipose tissue samples identified ~160 FAHFA regioisomers and QTL analysis highlighted several positional candidate genes in PAHSA metabolism. The results indicate that the synthesis of PAHSAs via carbohydrate-responsive element-binding protein (ChREBP)-driven de novo lipogenesis is linked to the adaptive antioxidant system and the remodelling of phospholipid hydroperoxides.
► Ondrej Kuda✉ On the Complexity of PAHSA Research. Cell Metabolism 2018, Sep 20; DOI https://doi.org/10.1016/j.cmet.2018.09.006
Comments on the methodological and conceptual problems when working with FAHFAs.
fulltext at https://www.cell.com/cell-metabolism/pdf/S1550-4131(18)30571-0.pdf
free fulltext link https://authors.elsevier.com/a/1Xq8i5WXUlA-Mk