The intracellular concentration of potassium and sodium cations, as well as of protons (pH), is strictly regulated via the activity of a series of membrane proteins that mediate the flux of cations and protons with various transport mechanisms. Incorrect functioning of some transporters results in serious disorders and diseases. We study in detail the roles of individual transporters and the impact of their activity on cell fitness.
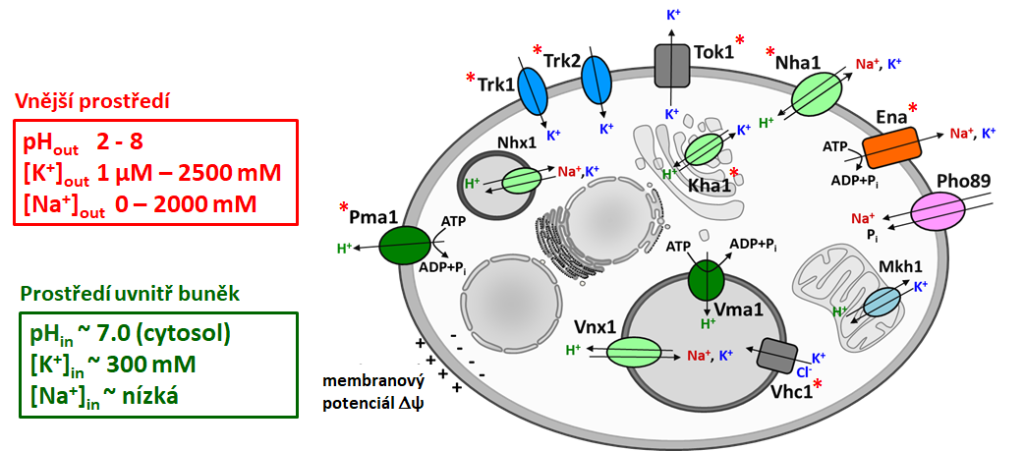
Potassium cations are crucial for many physiological processes (e.g. for negative charges compensations in macromolecules, for the regulation of intracellular pH, membrane potential, or cell volume). On the other hand, increased levels of other alkali-metal cations (Li+, Na+, Rb+) are toxic for cells. For the maintenance of optimal intracellular cation concentrations in the cytosol, cells use a whole range of transporters whose perfect coordination ensures the cell a stable intracellular environment indispensable for the maintenance of life.
The best-studied model of eukaryotic cells is the yeast Saccharomyces cerevisiae. It is able to survive in environments with various pH and it tolerates a broad range of cation concentrations. Inside the cell, in the cytosol, concentrations of K+ and Na+ cations must be tightly regulated. To cope with this, yeast cells possess more than ten different transporters for K+, Na+, and H+ localized in the plasma membrane (ensuring import and export) or in membranes of intracellular organelles (transporting cations between cytosol and cell organelles). By characterizing particular transporters, we try to understand how eukaryotic cells adapt to changes in ion concentrations in their environment.
Genes for particular transporters can be deleted in S. cerevisiae cells. Our laboratory possesses a large collection of yeast strains with deletions of genes for a single transport system or with combinations of these deletions. Yeast strains lacking their own transport systems serve us for heterologous expression and studying transporters from higher organisms (plant, animal or human).
Regarding the particular transporters, we are interested in their:
1. Localization
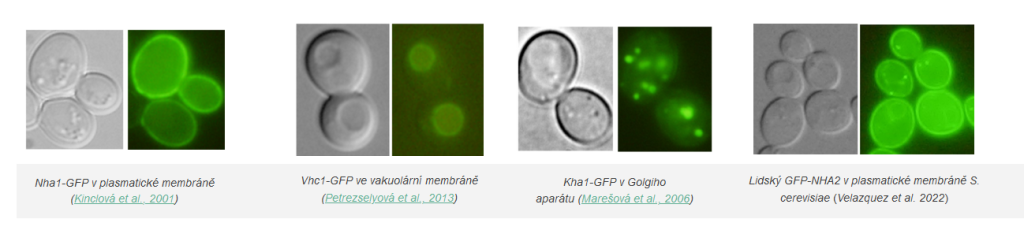
Studied proteins are tagged with fluorescent markers and cells producing these proteins are visualized by fluorescent microscope.
Examples of transporters’ localization in S.cerevisiae
2. Structure
By using mutagenesis, we search for amino-acid residues important for the transporter function.
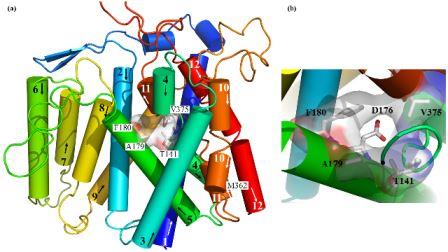
Structural (3D) model of the transmembrane part of Na+/H+ antiporter Sod2-22 from Z. rouxiibased on E. coli NhaA antiporter structure (Kinclova-Zimmermannova et al., 2015). (a) General side view of the model. Highlighted residues Thr141, Ala179, Phe180 and Val375 are important for the determination of substrate specificity of the transporter. (b) Detailed view of hydrophobic cation filter important for the determinaton of substrate specificity of yeast Na+/H+ antiporters. The 3D model is observed from the top among helices 2, 4, 5 and 11. The putative cation binding residue (Asp176) placed behind the hydrophobic filter is also shown.
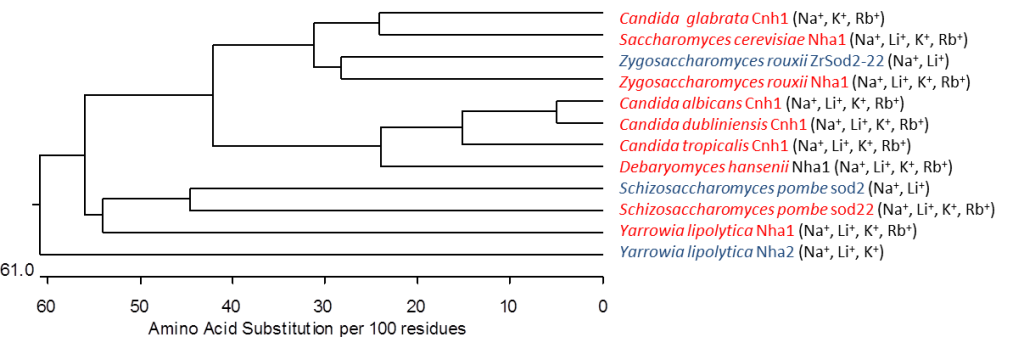
Characterization of the family of plasma membrane Na+/H+ antiporters from lower and higher eukaryotes
The family of yeast plasma membrane Na+/H+ antiporters includes transporters that transport all alkali-metal cations (Li+, Na+, K+, Rb+), but also systems that recognize only Li+ and Na+ (Kinclová et al., 2002). We have identified amino-acid residues important for recognition, binding and transport of cations in antiporters in the plasma membrane of yeast cells (S. cerevisiae Nha1, Zygosaccharomyces rouxii Sod2-22) (Kinclova-Zimmermannova et al., 2005; 2006; 2015; Albacar et al. 2021) as well as in human antiporter NHA2 (Vélazquez et al. 2022). We created 3D-model of Sod2-22 transmembrane part that revealed that amino-acid residues identified by mutagenesis form a hydrophobic filter in the putative cation pathway’s core which is important for the determination of substrate specificity of Na+/H+ antiporters (Kinclová-Zimmermannová et al., 2015). Currently, we try to identify other protein regions that determine substrate specificity and transport activity in Na+/H+ antiporters, and also in other types of cation transporters.
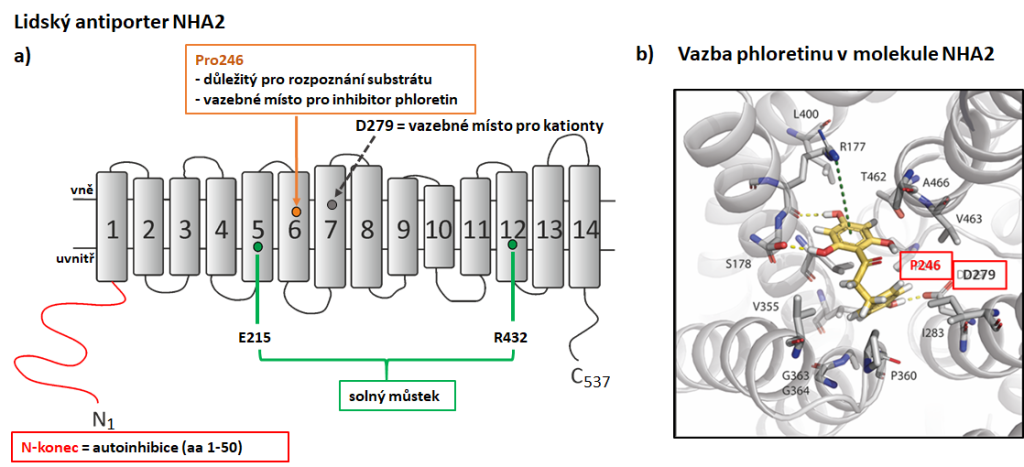
NHA2 performs multiple functions in the human body 71 and its malfunctioning leads to various pathologies ranging from metabolic to fertility disorders. a) A topology model of NHA2 structure with 14 transmembrane segments. Important regulatory regions and their significance for the function of the transporter that we identified by using a heterologous expression of NHA2 in S. cerevisiae are indicated. b) A model of binding of NHA2-specific inhibitor phloretin to the NHA2 molecule. We discovered that phloretin binds close to the cation-binding site (D279). A substitution of P246 for a polar amino acid threonine or serine decreased the inhibition effect of phloretin (Vélazquez et al. 2022).
3. Transport properties
We determine transporter substrate specificity and transport activity.
H+ antiporters that have been studied in our laboratory. Blue highlights antiporters recognizing as their substrates only or with a significantly higher affinity Na+ and Li+ cations; they primary detoxify yeast cells. Red highlights antiporters exporting potassium cations whose function in cell physiology is more complex – they participate in the regulation of intracellular K+, internal pH and cell volume.
4. Activity regulation
Transporters are regulated at the level of gene expression, biogenesis of the protein or via interaction with other proteins. We determined various new regulatory mechanism that participate in the maintenance of cation and pH homeostasis in eukaryotic cells, e.g. at the level transporters’ biogenesis (Rosas-Santiago et al., 2015, 2016; 2017; Zimmermannova et al. 2019), via tight connection among transporters of protons, potassium and/or calcium cations (Zimmermannova at al., 2015; Papouskova et al., 2015; Zimmermannová et al. 2021) or via the lipid composition of cell membranes (Kodedová et al., 2015). We have found that regulatory proteins 14-3-3 (Smidova et al. 2019) or phosphatase Ppz1 (Albacar et al. 2021) also participate in the regulation of yeast cation transporters’s activity.
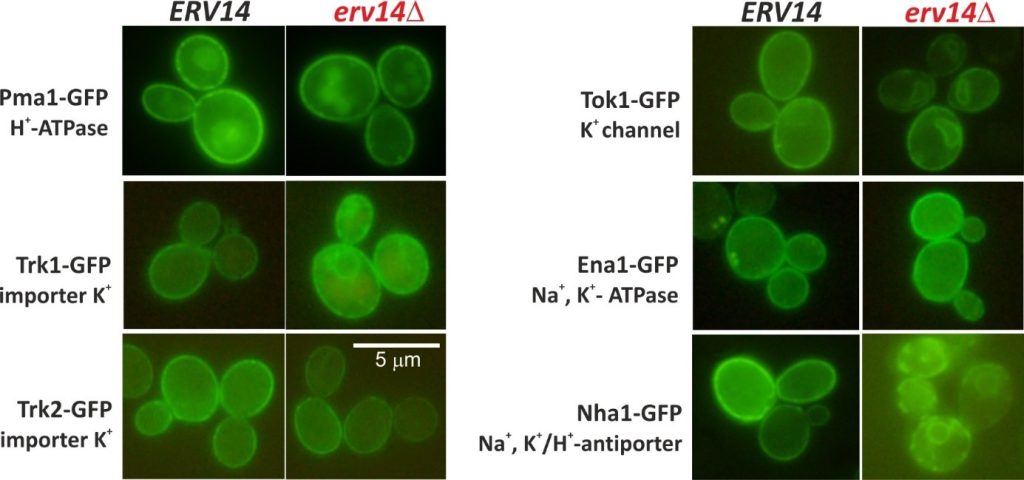
The biogenesis of potassium transporters Trk1, Tok1 and Nha1 depends on the presence of the Erv14/cornichon cargo receptor. Deletion of the ERV14 gene results in stacking of these transporters in the endoplasmic reticulum. On the other hand, trafficking of Pma1, Trk2 or Ena1 transporters to the plasma membrane is Erv14–independent (Rosas-Santiago et al. 2016, Zimmermannova et al. 2019). Four isoforms of Erv14 protein exist in human cells, CNIH1-4. Currently, we study properties of CNIH proteins, their ability to complement function of the yeast homolog, and their possible involvement in biogenesis of human as well as yeast transporters.