The transcription factor p53 is a key regulator of apoptosis, senescence and DNA repair, which protects cells against tumorigenesis under various cellular stresses. The functions of p53 are closely intertwined with the activity of Forkhead box O (FOXO) transcription factors.
Biophysical studies
Here, we characterize the interaction between p53 and FOXO4 by NMR, chemical cross-linking, and analytical ultracentrifugation.Our results reveal that the interaction between p53 TAD and the FOXO4 Forkhead domain is essential for the overall stability of the p53:FOXO4 complex. Furthermore, contacts involving the N-terminal segment of FOXO4, the C-terminal negative regulatory domain of p53 and the DNA-binding domains of both proteins stabilize the complex, whose formation blocks p53 binding to DNA but without affecting the DNA-binding properties of FOXO4. Therefore, our structural findings may help to understand the intertwined functions of p53 and FOXO4 in cellular homeostasis, longevity, and stress response.
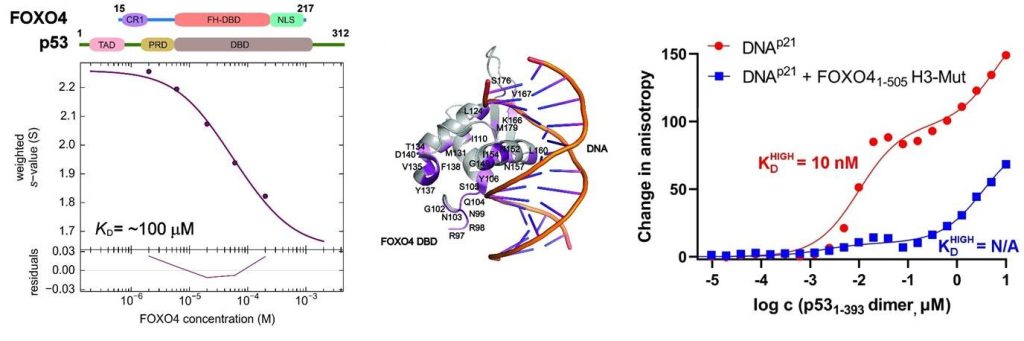
Left, sedimentation velocity analytical ultracentrifugation analysis of interaction between FOXO4 and p53. Middle, chemical shift perturbations obtained from 1H-15N HSQC spectra of 15N-labeled FOXO4 in the presence of p53 mapped onto the crystal structure of the FOXO4 DBD:DNA complex. Right, fluorescence anisotropy measurements showing that the complex formation reduces the DNA-binding affinity of p53 (Mandal et al. (2022) Protein Science).