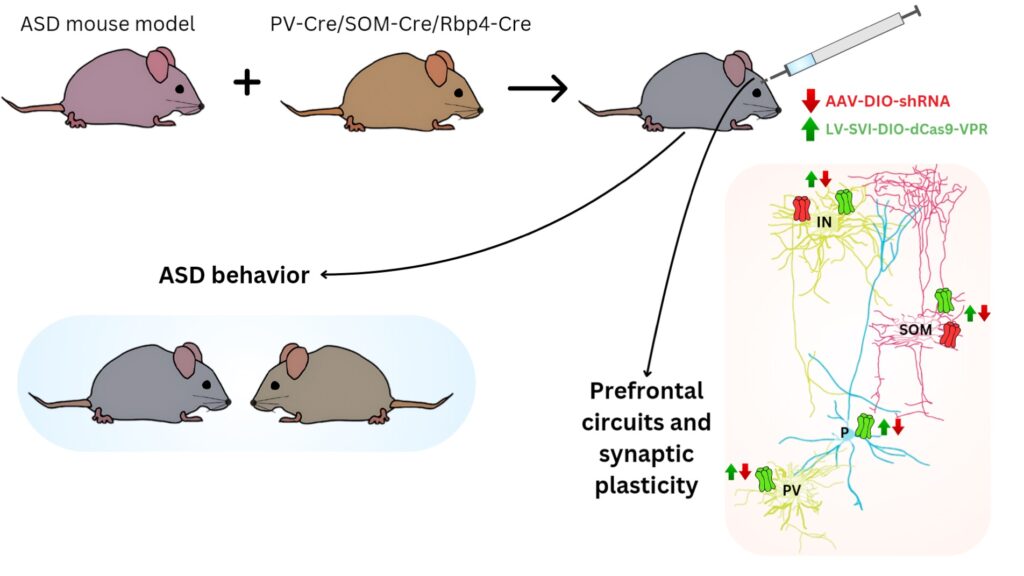
The main goal of this project is to determine which neuronal populations expressing nAChRs in the prefrontal cortex can represent the ideal target for the reversal of behavioral symptoms present in patients with autism spectrum disorder (ASD) such as social impairment, aggression and cognitive inflexibility
nAChRs have been associated with various neuropsychiatric disorders, including autism spectrum disorder (ASD). ASD patients show a markedly decreased probability of heavy smoking and nicotine addiction compared to the general population. Studies in ASD patients have indicated that alterations in the CHRNA7 gene, coding for the alpha7 nicotinic subunit, are linked to intellectual disability, attention deficit and ASD. Because of the suspected role of nicotinic signaling in ASD, several clinical trials have been launched to test the effect of nicotine and other nicotinic ligands in ASD patients. In preclinical studies, mouse models of autism showed a reversal of social deficit and repetitive behavior after the administration of nicotine and other nicotinic ligands. However, mechanisms underlying these effects have not yet been identifie
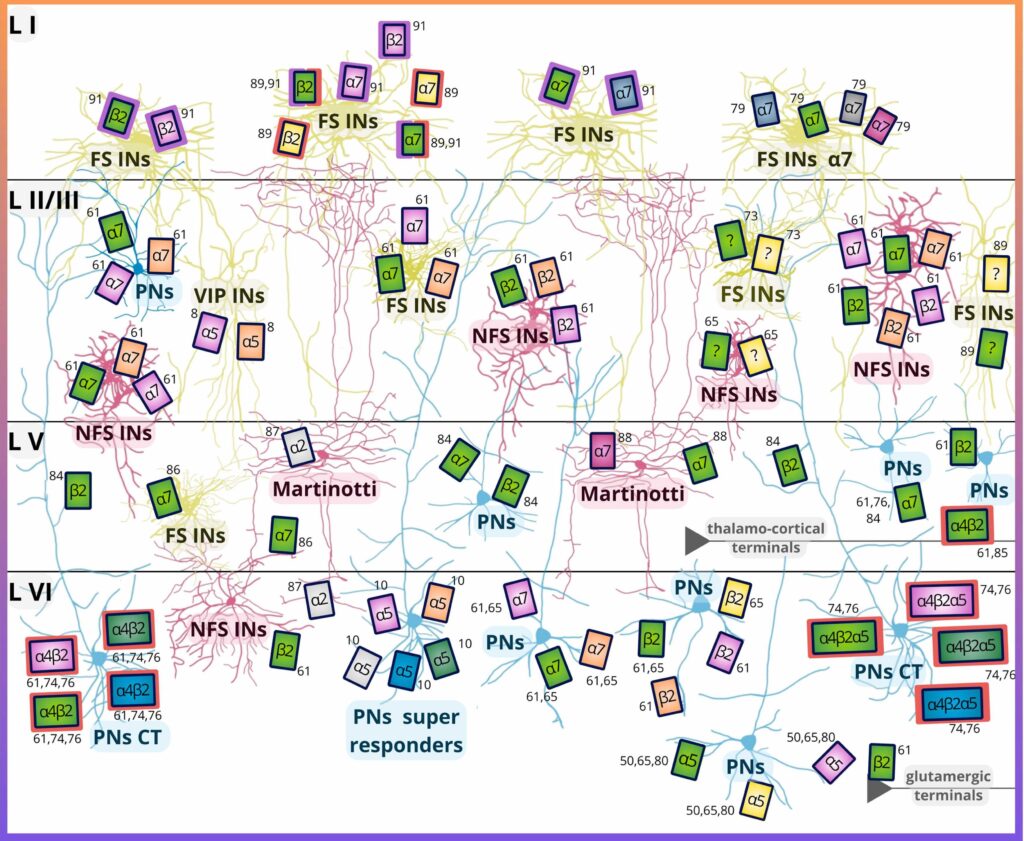
Various subtypes of nicotinic acetylcholine receptors are expressed by neuronal populations in
different layers of the prefrontal cortex. Main neuronal populations of the prefrontal cortex are shown
including pyramidal neurons (PN), fast-spiking (FS) and non-fast-spiking (NFS) interneurons.
The prefrontal cortex (PFC) is one of the brain regions that play an important role in ASD pathophysiology. In the PFC, nAChRs are widely but specifically expressed by various neuronal populations and the expression pattern also changes based on the different cortical layers and nicotinic subunits. In certain layers of the PFC, the same nAChR subtypes are expressed by excitatory pyramidal neurons as well as by inhibitory interneurons, these two often having antagonistic effects on local circuits.
We hypothesize that specific activation of nAChRs expressed selectively by PFC interneurons would lead to the strengthening of inhibitory input in the PFC and therefore would be more efficient (and also safer) compared to general nicotinic activation. The goal of this project is to identify a PFC neuronal population whose activity can be modulated through nAChRs, leading to a reversal of typical ASD behavioral symptoms such as impairment of social behavior, aggression and cognitive inflexibility. To achieve this goal, we are using mouse models of ASD crossed with other genetic mouse models which allows us to decrease or increase the expression of nAChRs only in selected neuronal populations. Then we evaluate the effect of nAChR modulation on ASD-like behavior, neuronal activity in the PFC and synaptic plasticity.
