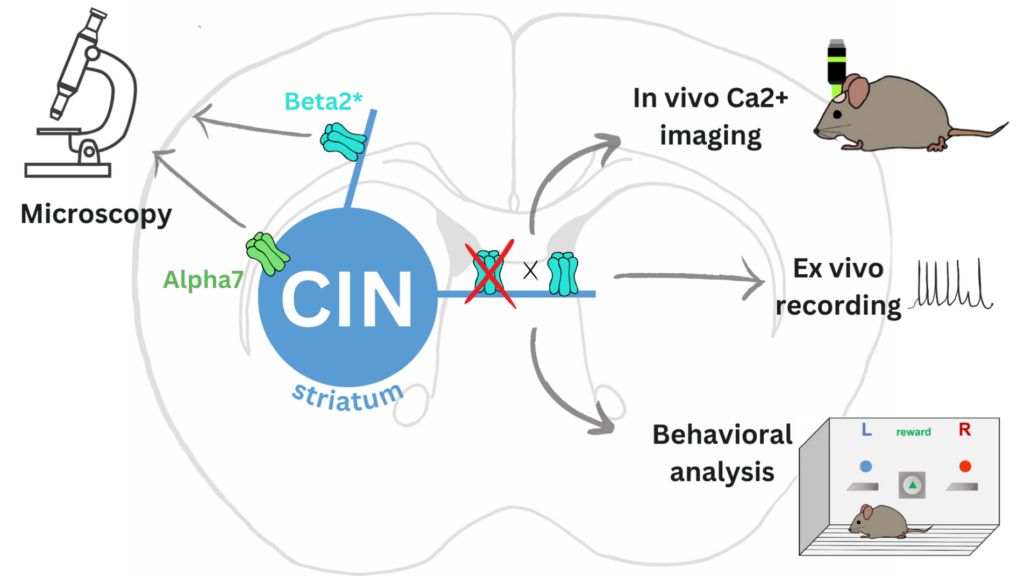
The ultimate goal of this project is to obtain a mechanistic understanding of how nAChRs expressed by striatal cholinergic interneurons (CINs) control the functioning of these neurons and through this, the activity of striatal circuits and animals’ behavior.
Striatum is a peculiar structure in many aspects, including nicotinic expression. While there is a high level of nAChR expression in the striatum, most of these receptors are present in axon terminals projecting to the striatum from other brain regions. Most striatal resident neurons do not express nAChRs at all and striatal nAChRs are only present in specific and rare types of striatal interneurons including CINs. The scarcity of nAChRs in striatal neurons led us to question whether these receptors have any functional significance and, if yes, how powerful and specific is the effect of their activation. Can we use them as a specific tool to modulate the activity of CINs and the striatal-associated pathological symptoms such as impairment of motor functions in Parkinson’s disease or decision-making in addiction?
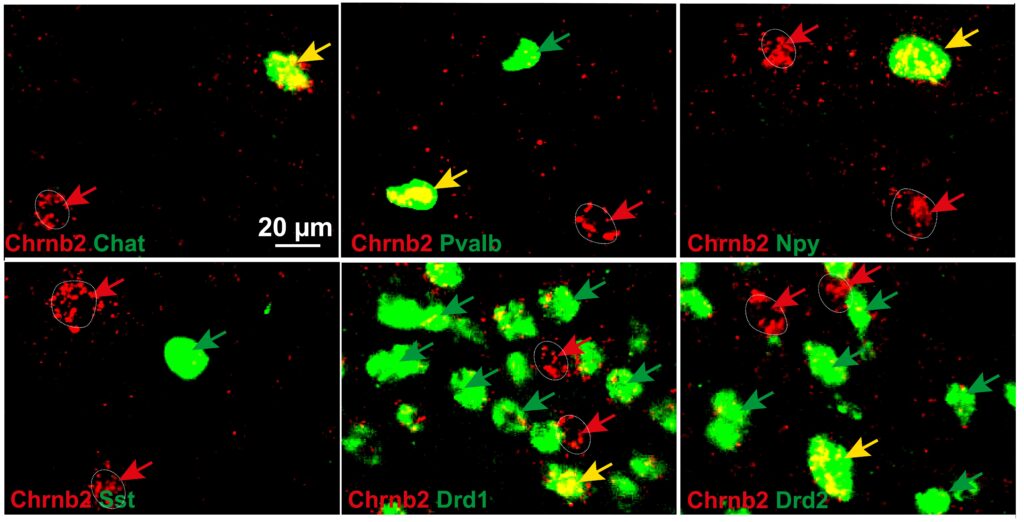
Expression of beta2 nicotinic subunit in different types of striatal neurons. Beta2 subunit is
shown in red and markers for individual neuronal types are shown in green.
To answer these questions, we need to first identify the subunit-specific subtypes of nAChRs that are expressed by CINs. To better understand their function, we also investigate the subcellular localization of individual nAChRs in CINs. We abolish the function of nAChRs in CINs using genetically modified mouse lines and viral vectors and then investigate the resulting neuronal and behavioral changes. We have already discovered that CINs express heteromeric beta2-containing nAChRs and that the deletion of these receptors has marked functional consequences. It leads to an increase in the activity of striatal projection neurons and on the behavioral level, it increases sensitivity to stimulant drugs and affects social, anxiety-like and explorative behavior.
To further uncover the role of nAChRs in controlling of CINs and striatal circuits, we now plan to use in vivo Ca2+ imaging in freely behaving animals and see how the deletion of nAChRs in CINs changes striatal signaling during behavior.