We use various methods, e.g. estimation of the membrane potential to study the impact of the membrane lipid composition on the sensitivity of yeast cells to antifungal drugs (Kodedová and Sychrová, 2015; Kodedová et al., 2019; Csáky et al., 2020) by determination of changes in plasma-membrane potential and other methods.
The yeast cell membrane usually contains ergosterol and no cholesterol, which is the main sterol present in animal cells. Ergosterol is necessary for the regulation of membrane permeability and fluidity, and for regulating the activity of membrane transporters. Thus, due to its indispensable role in yeast physiology, ergosterol and its biosynthesis became major targets in developing antifungal drugs. Under special conditions, mammalian cholesterol can substitute for ergosterol. Some pathogenic yeasts, such as Candida glabrata and C. krusei, import exogenous cholesterol from the host serum and incorporate it into their membranes to compensate for ergosterol depletion caused by sterol biosynthesis inhibitors and to protect themselves against polyenes such as amphotericin B. We study this resistance mechanism using as a model S. cerevisiae cells, which become sterol auxotrophs in the absence of oxygen or with hem1∆ mutation, and import cholesterol and other sterols to their plasma membrane.
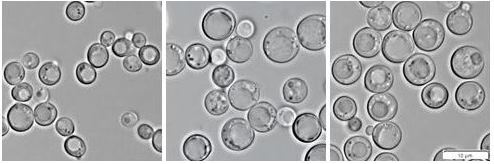
Different morphology and size of hem1Δ cells cultivated in the presence of ergosterol (left), cholesterol (middle), and intermediate of cholesterol biosynthesis 7-dehydrocholesterol (right panel).
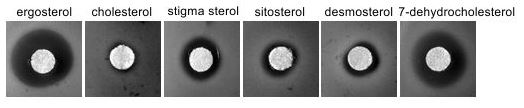
Resistance of hem1∆ cells accumulating alternative sterols to polyene nystatin compared to cells accumulating ergosterol.
We aim to elucidate the mechanism of sterol transport in yeast cells to demonstrate how the sterol composition of the cell membranes contributes to antifungal resistance.









