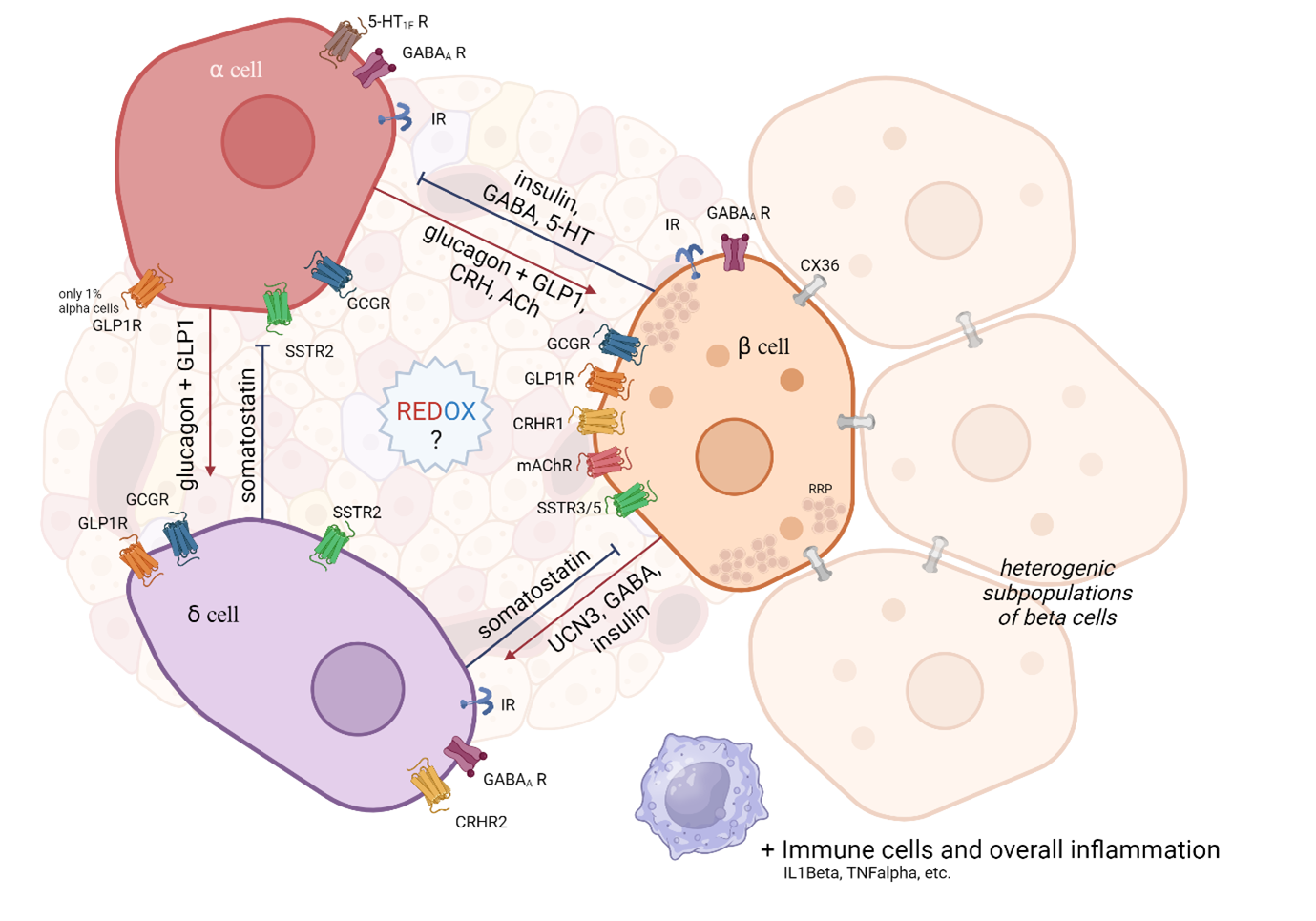
This project aims to understand how disruptions in the redox state contribute to the dysfunction of islet cells and the development of metabolic disorders such as type 2 diabetes. While much is known about beta-cell dysfunction in diabetes, the roles of other endocrine cells in the development of metabolic diseases remain poorly understood. We focus on the role of the redox state in intercellular communication in pancreatic islets. These are mainly composed of three types of endocrine cells: alpha (glucagon production), beta (insulin production), and delta (somatostatin production).
Abbreviations used: IR – insulin receptor, GCGR – glucagon receptor, GLP1R – glucagon-like peptide 1 receptor, SSTR – somatostatin receptor (isoforms 2,3,5), GABA? R – GABA type A receptor (inhibitory neurotransmitter), 5-HT1F R – Serotonin receptor type 1F, CRHR – Corticotropin-releasing hormone receptor, mAChR – Muscarinic acetylcholine receptor, CX36 – Connexin 36, RRP – Ready releasable pool.
Proper communication within the islet (paracrine and autocrine) and with peripheral tissues (incretins from the digestive system, nervous system, immune system, etc.) is essential for maintaining appropriate blood glucose regulation. Alpha, beta, and delta cells communicate with each other using their own hormones and other signaling substances (see figure) by acting through specific receptors on the surface of target cells. The most attention is directed to the majority population of beta cells. This is further subdivided into other subpopulations, e.g. ‘pacemaker-like Hubb’ cells generating an action potential wave that triggers a synchronous discharge from surrounding cells via gap junctions (CX36).
Insulin secretion from beta cells is stimulated by increased glucose metabolism, leading to changes in the redox environment and reversible modifications of sensitive proteins (oxidation, acetylation, phosphorylation) (Holendova et al, 2024). Specifically, we are studying a major source of reactive oxygen species (ROS) in beta cells, the enzyme NADPH oxidase 4 (NOX4), which has been described as a key signal amplifier in glucose-stimulated insulin release (Plecita-Hlavata et al, 2020). An imbalance in the redox environment is associated with impaired beta cell function. Deletion of Nox4 in beta-specific Nox4-/- mutant mice leads to a pro-reduction phenotype. These mice exhibit impaired biphasic insulin secretion and peripheral insulin resistance. Conversely, increased NOX4 activity and a pro-oxidant beta cell environment due to metabolic stress leads to increased systemic inflammation, e.g. on a high-fat diet (Holendova et al, 2024). It is not yet clear how other endocrine cells play a role in the development of health complications. Their involvement in adulthood is particularly important in the development of type 2 diabetes, where various compensatory mechanisms occur that have not yet been fully described.
References:
Holendová B, Šalovská B, Benáková Š, Plecitá-Hlavatá L. Beyond glucose: The crucial role of redox signaling in β-cell metabolic adaptation. Metabolism 2024;161:156027.
Lydie Plecitá-Hlavatá, Martin Jabůrek, Blanka Holendová, Jan Tauber, Vojtěch Pavluch, Zuzana Berková, Monika Cahová, Katrin Schröder, Ralf P. Brandes, Detlef Siemen, Petr Ježek; Glucose-Stimulated Insulin Secretion Fundamentally Requires H2O2 Signaling by NADPH Oxidase 4. Diabetes 1 July 2020; 69 (7): 1341–1354. https://doi.org/10.2337/db19-1130
Holendová B, Benáková Š, Křivonosková M, Pavluch V, Tauber J, Gabrielová E, Ježek P, Plecitá-Hlavatá L. NADPH oxidase 4 in mouse β cells participates in inflammation on chronic nutrient overload. Obesity (Silver Spring) 2024;32:339-351.