Kardiometabolismus

Na této straně najdete
O oddělení
Heart disease remains the leading cause of death worldwide, with striking differences in symptoms, prevalence, and outcomes between males and females. As such, biological sex represents an important biological variable that must be studied, understood and integrated into both basic and clinical research. Advancing this understanding holds the potential to develop more precise and effective therapies that benefit all patients equally.
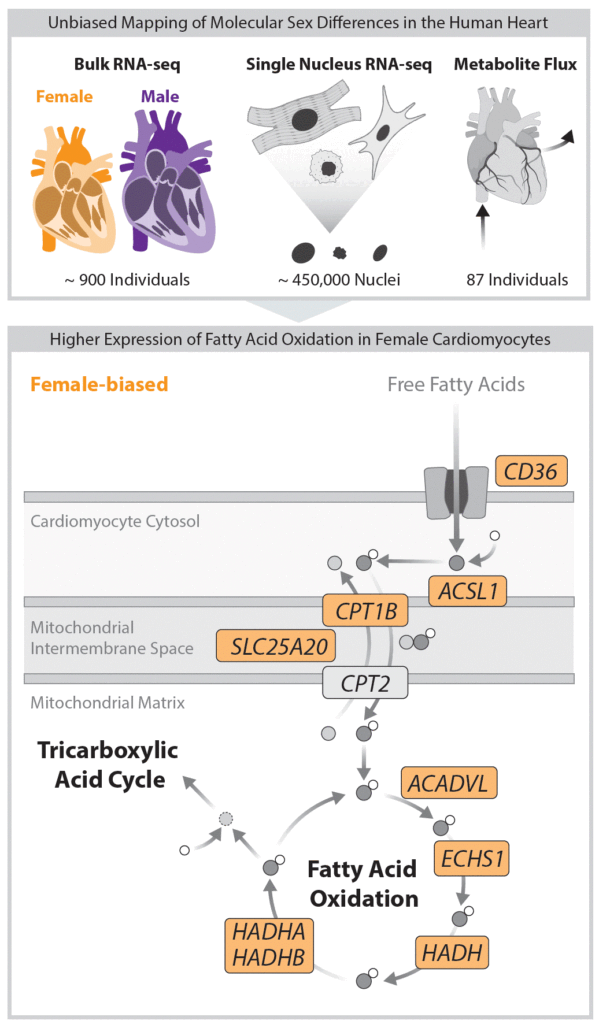
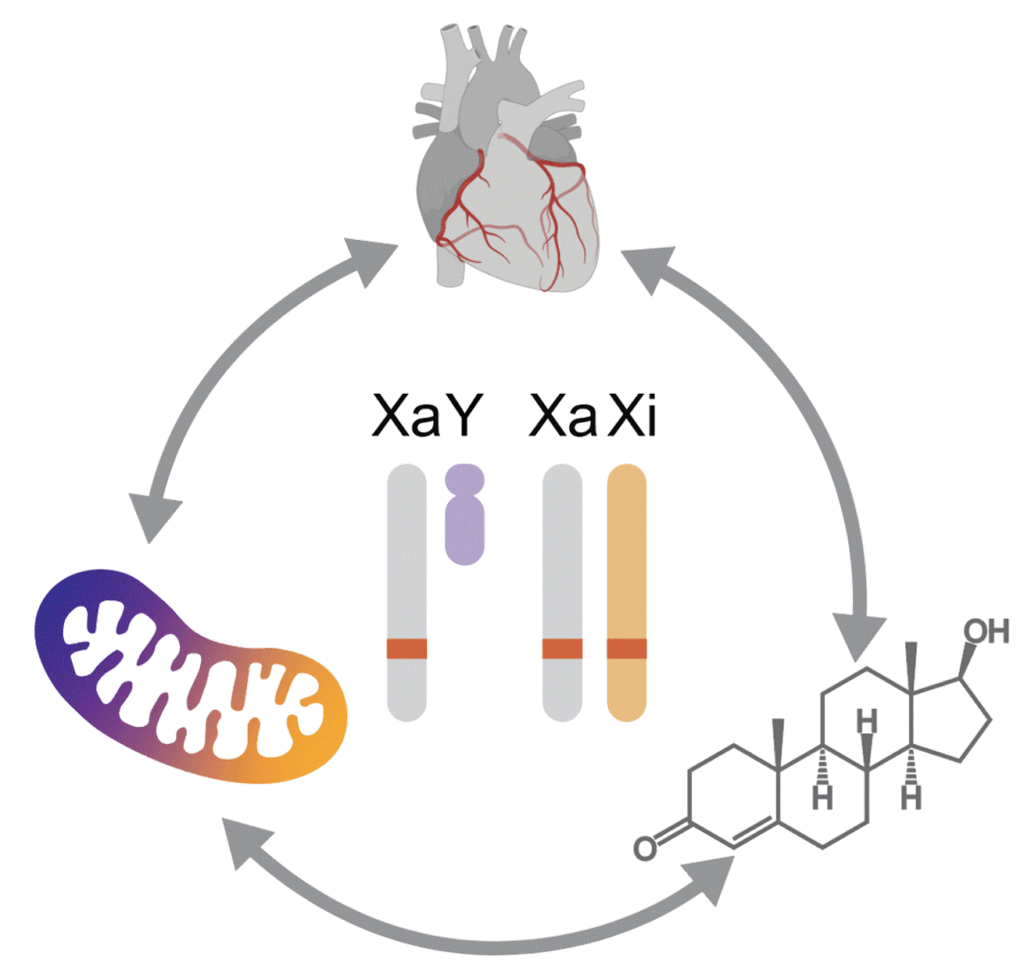
Our recent study (Talukdar & Chmatal, Circulation, 2025) demonstrated a key role of biological sex – defined by a unique combination of sex chromosomes and gonadal hormones – in shaping mitochondrial function in non-failing human heart. Specifically, we found that genes involved in mitochondrial fatty acid oxidation – a central pathway in cardiac energy production – are expressed at higher levels in female cardiomyocytes than in males. Given that mitochondria are highly responsive to cell-specific nutritional and physiological cues, this raises a fundamental biological question: What molecular and genetic mechanisms allow heart mitochondria to adapt to the distinct metabolic demands of females compared to males?
My newly established research group is dedicated to uncovering the molecular basis of sex differences in cardiac metabolism and mitochondrial biology, both in health and disease. Our work focuses on the role of sex chromosomes, whose contribution remains largely unexplored – presenting exciting opportunities for novel scientific insights. To address this challenge, we take an integrative approach that combines systems biology, advanced omics technologies (transcriptomics, proteomics, metabolomics), and cell biology techniques, using both genetically engineered mouse models and human samples.
Our research focuses on two major goals:
- To define how biological sex shapes healthy cardiac metabolism across multiple levels – ranging from the whole organism to cardiac tissue, individual heart cells, and their mitochondria.
- To dissect the molecular mechanisms driving these sex-specific differences and understand how these processes are integrated across organelles, cells, and tissues.
We are actively recruiting motivated and curious Ph.D. students and postdoctoral researchers who are excited to make discoveries in this dynamic and impactful field. If you’re passionate about mitochondrial biology, cardiac metabolism, or sex differences in health and disease, we would love to hear from you! Interested candidates should send a CV, a motivation letter, and contact information for three referees to: lukas.chmatal@fgu.cas.cz
Maya Talukdar*, Lukas Chmátal*, Linyong Mao, Daniel Reichart, Danielle Murashige, Yelena Skaletsky, Daniel M. DeLaughter, Zoltan Arany, Jonathan G. Seidman, Christine Seidman, David C. Page. Genes of fatty acid oxidation pathway are upregulated in the female as compared to male human cardiomyocytes. Circulation, (2025) *co-first authors
Daniel Reichart, Gregory A. Newby, Hiroko Wakimoto, Mingyue Lun, Joshua M. Gorham, Justin J. Curran, Aditya, Raguram, Daniel M. DeLaughter, David A. Conner, Júlia D. C. Marsiglia1, Sajeev Kohli, Lukas Chmátal, David C. Page, Nerea Zabaleta, Luk Vandenberghe, David R. Liu, Jonathan G. Seidman, and Christine Seidman. Efficient in vivo genome editing prevents hypertrophic cardiomyopathy in mice. Nature Medicine, 29: 412-421 (2023)
Godfrey A.K., Naqvi S., Chmátal L., Chick J.M., Mitchell R.N., Gygi S.P., Page D.C. Quantitative analysis of Y-chromosome gene expression across 36 human tissues. Genome Research, 30: 860-873 (2020)
Akera T., Chmátal L., Trimm E., Yang K., Aonbangkhen C., Chenoweth D.M., Janke C., Schultz R.M., Lampson M.A. Spindle asymmetry drives non-Mendelian chromosome segregation. Science, 358(6363): 668-672 (2017)