The temperature-sensitive subset of the vanilloid transient receptor potential channel family includes TRPV1-TRPV4 channels that respond to temperatures ranging from moderate (25 °C) to noxious (>50 °C). Their important roles in various physiological and pathological processes, such as pain sensation, inflammation, skin homeostasis, osmotic regulation and diverse cardiovascular, neurological, respiratory, renal and metabolic functions, make these channels prominent pharmacological targets.
However, understanding the structural basis of their activation is still a major challenge. Bound membrane lipids have been shown to play an active role in temperature-dependent gating of TRPV3 [1]. Unlike other ligand-gated ion channels that are activated from only one agonist binding site, thermoTRPV channels are capable of activation by various stimuli of different modalities. Direct inhibition of TRPV1 by antagonists shows significant analgesic effects, but due to the channel’s involvement in body temperature regulation, it also has serious side effects such as hyperthermia, which is why all TRPV1 inhibitors have so far failed in clinical trials. In contrast, TRPV1 vanilloid agonists are used for very effective local pain control, which first open the channels, and then desensitize the channel itself as well as the nociceptors by inducing calcium overload. However, this latter approach can lead to irreversible inactivation of nociceptors and is therefore currently only being considered for use in intractable cancer pain (ClinicalTrials.gov: NCT05067257). Therefore, despite the great attention paid to the development of new compounds toward thermoTRPVs in the last 30 years, novel strategies for the reversible pharmacological up- and down-regulation of these channels are urgently needed.
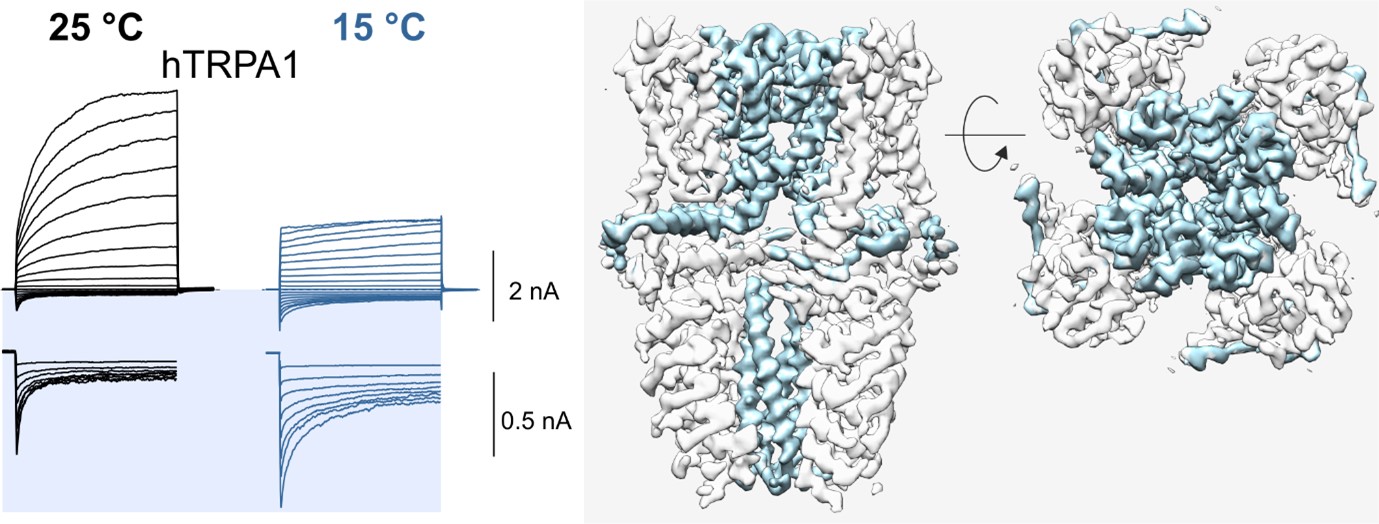
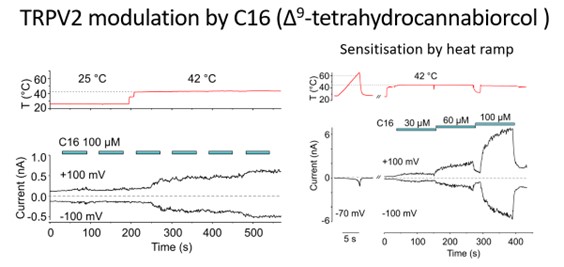
This project addresses the effect of several selected compounds whose interaction with TRP receptors modulates the binding site for orthosteric agonists so that the temperature sensitivity of these receptors is not affected. It builds on our previous work and collaboration with structural biologists from Lund University and University of Copenhagen. Together we previously showed that the thermoTRP channel, specifically TRPA1, can gain cold sensitivity and lose heat sensitivity by truncating the N-terminus along with a voltage sensor-like domain (VSLD) [2]. In addition, responses to cold were also modulated by the replacement of two cysteines located in a short helix at the cytosol-membrane interface located near the VSLD. We have also recently revealed that probenecid acts as a strong positive allosteric modulator of TRPV2 and identified a new cannabinoid binding site within the vanilloid pocket that is under the influence of allosteric control by this compound [3].
[1] Nadezhdin, K.D., Neuberger, A., Trofimov, Y.A., Krylov, N.A., Sinica, V., Kupko, N., Vlachova, V., Zakharian, E., Efremov, R.G. and Sobolevsky, A.I., 2021. Structural mechanism of heat-induced opening of a temperature-sensitive TRP channel. Nature structural & molecular biology, 28(7), pp.564-572.
[2] Moparthi, L., Sinica, V., Moparthi, V.K., Kreir, M., Vignane, T., Filipovic, M.R., Vlachova, V. and Zygmunt, P.M., 2022. The human TRPA1 intrinsic cold and heat sensitivity involves separate channel structures beyond the N-ARD domain. Nature communications, 13(1), p.6113.
[3] Zhang, L., Simonsen, C., Zimova, L., Wang, K., Moparthi, L., Gaudet, R., Ekoff, M., Nilsson, G., Hellmich, U.A., Vlachova, V. and Gourdon, P., 2022. Cannabinoid non-cannabidiol site modulation of TRPV2 structure and function. Nature Communications, 13(1), p.7483.