Laboratory of Mitochondrial Physiology

Content of this page
About the Laboratory
Mitochondrial metabolism involves canonical energy production as well as non-canonical functions, specifically metabolic signaling, redox signaling, or epigenetic regulation. Both canonical and non-canonical mitochondrial functions are tissue-specific, activated under various stress stimuli, or nutrient starvation. Our projects focus on mitochondrial metabolic pathways that can induce complex changes in redox signaling, mitochondrial and cellular metabolic landscape, and associate with pathological states, such as type 2 diabetes or cancer progression. Our main projects are:
- Redox biology of insulin secretion and in development of type 2 diabetes
- Mitochondrial ultramorphology
- Specificities of cancer metabolism
- Development of GLP-1-conjugated nanoparticles for β-cell magnetic resonance imaging
Projects
Achievements
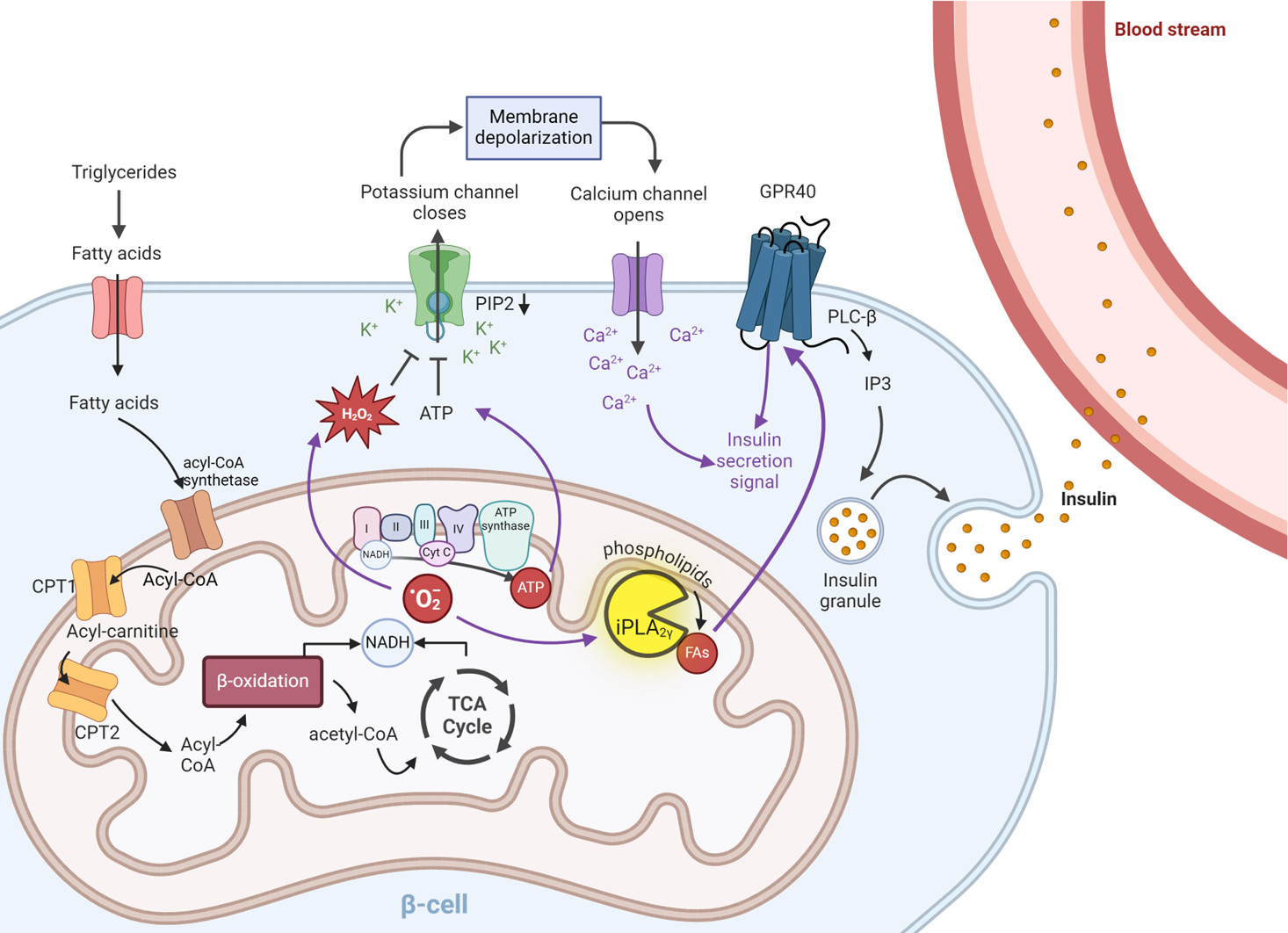
Mitochondria to plasma membrane redox signaling is essential for fatty acid β-oxidation-driven insulin secretion
Jabůrek, M., Klöppel, E., Průchová, P., Mozheitova, O., Tauber, J., Engstová, H., & Ježek, P. (2024). Mitochondria to plasma membrane redox signaling is essential for fatty acid β-oxidation-driven insulin secretion. Redox Biology, 75, 103283. https://doi.org/10.1016/j.redox.2024.103283
β-cells function as crucial ‘fuel sensors’, actively monitoring and responding to elevated nutrient loads by releasing insulin. Mitochondria are essential for the proper functioning of pancreatic β-cells, playing a key role in the generation of metabolic coupling factors such as ATP, which ultimately participate in the closure of ATP-sensitive K+ channels. This closure results in depolarization of the plasma membrane, which activates voltage-gated calcium (CaV) channels, allowing Ca2+ influx. The increased concentration of free Ca2+ in the cytosol triggers the release of insulin-containing granules through exocytosis. However, some authors propose that this model is incomplete, suggesting that additional components may be involved in signal amplification and insulin release.
While the primary trigger for insulin release is high blood glucose levels, other nutritional factors—including specific amino acids, keto acids, certain hormones, and fatty acids—can also enhance pancreatic insulin secretion. In this study, we focus on fatty acid-stimulated insulin secretion (FASIS). We have demonstrated that redox signaling is crucial for FASIS at non-stimulating glucose levels. Furthermore, we found that an elevated ATP/ADP ratio alone is insufficient to trigger insulin secretion.
We propose a mechanism for FASIS that highlights the role of mitochondrial phospholipase iPLA2γ in the regulation of insulin secretion. Under non-stimulating glucose conditions, an increase in free fatty acids enhances β-oxidation, leading to increased mitochondrial oxidant production and activation of iPLA2γ. iPLA2γ then catalyzes the hydrolysis of phospholipids, producing free fatty acids. These endogenous mitochondrial fatty acids function as preferred agonists of GPR40. This sequence of events stimulates Ca²⁺ release from intracellular stores, ultimately promoting insulin secretion. Our experiments using iPLA2γ knockout mice provide further support for this hypothesis.
In conclusion, we establish a connection between redox signals generated from β-oxidation, the activation of iPLA2γ, and the release of insulin in pancreatic β-cells.
Dedicated to grants of the Czech Science Foundation (GAČR): No. 20-00408S, 21-01205S, 24-10132S, all to P.J. and 22-17173S to M.J; plus to CarDia, i.e. the project “National Institute for Research of Metabolic and Cardiovascular Diseases” (Programme EXCELES, ID Project No. LX22NPO5104) – funded by the European Union – Next Generation EU.
BCAA metabolism in pancreatic cancer affects lipid balance by regulating fatty acid import into mitochondria
https://doi.org/10.1186/s40170-024-00335-5
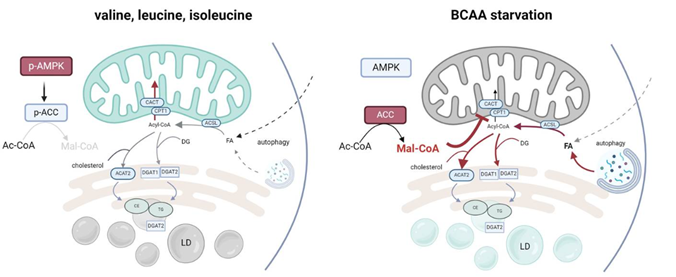
Branched-chain amino acids (BCAAs) are essential amino acids catabolism of which takes place in mitochondria. We currently acknowledge the role of BCAAs in transamination, complete oxidation, and as metabolic precursors, i.e. cholesterol, and certain types of fatty acids (FAs). Besides this, leucine have been implicated in the activation of mammalian target of rapamycin complex 1 (mTORC1) signaling. The interference of BCAA catabolism with other metabolic pathways is unexplored and the consequences of such crosstalk remain completely unknown. Hence, we performed an in-depth analysis of BCAA metabolism in pancreatic cancer cells in order to reveal specificities of BCAA utilization in pancreatic cancer-derived cells.
During tumor growth levels of nutrients in tumor microenvironment might be decreased or completely exhausted. According to our data, starvation of BCAA significantly impacts cell growth and bioenergetics capacity of PDAC cells. Although BCAAs are not completely oxidized in PDAC cells, BCAA metabolism likely interferes with other anabolic and catabolic pathways.
The main finding of our work is that PDAC cells respond to BCAA starvation by DGAT1-dependent triacylglycerol (TG) synthesis and lipid droplet (LD) biogenesis. Increased TG synthesis and subsequent LD biogenesis under BCAA-depleted conditions follow autophagy onset, which is triggered by BCAA depletion, leading to decomposition of cellular membranes and generate free fatty acids (FAs), which subsequently esterify diacylglycerols.
The mechanism of free FA redirection into TG synthesis encompasses the inhibited import of cytosolic FAs into mitochondria. This phenotype is driven by the inhibited synthesis of long-chain carnitines (LC-CARs) due to alleviated 5′ AMP-activated protein kinase (AMPK) signaling. Reduced AMPK signaling activates acetyl-CoA carboxylase (ACC) and inhibits carnitine palmitoyltransferase 1A (CPT1) by enhanced malonyl-CoA production. So, the adaptive mechanism of PDAC cells to BCAA depletion includes a suppression of FA oxidation in mitochondria, which results in FA redirection into TGs and LDs.
We conclude that a decreased consumption of BCAAs can regulate the flux of FAs into β-oxidation and protects against mitochondrial substrate overload. We propose that restricting FA import into mitochondria protects cells from mitochondrial lipotoxicity, and this mechanism could represent a universal response to amino acid metabolism disturbances during cancer growth, regulating FA handling and storage. Our study provided a novel insight into the mitochondrial-cytosolic crosstalk mediated by metabolic signaling.
The study was supported by the grant NV19-01–00101 of the Czech Health Research Council and the institutional support RVO:67985823.
Novel mechanism of lipid droplets formation. Inhibited import of fatty acids into mitochondria during starvation of BCAA in PDAC cells leads to redirection of activated fatty acids into lipid droplets and protects against mitochondrial substrate overload. Created with BioRender.com
Two mitochondrial DNA polymorphisms modulate cardiolipin binding and lead to synthetic lethality
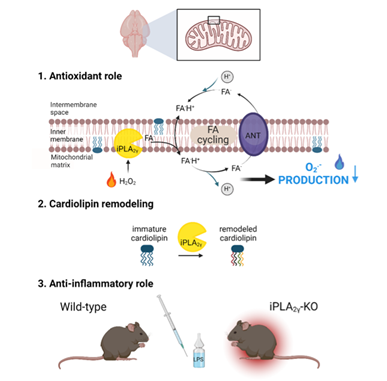
Průchová, P., Gotvaldová, K., Smolková, K., Alán, L., Holendová, B., Tauber, J., Galkin, A., Ježek, P., & Jabůrek, M. (2022). Antioxidant role and cardiolipin remodeling by redox-activated mitochondrial Ca2+-independent phospholipase A2gamma in the brain. Antioxidants, 11(2), 198. https://www.mdpi.com/2076-3921/11/2/198 or https://doi.org/10.3390/antiox11020198
Redox regulation plays a crucial role in a wide range of biological processes. Mitochondria in numerous tissues represent a primary source of superoxide and subsequent downstream oxidants, notably H2O2 and lipid hydroperoxides. However, the understanding of the role of mitochondrial oxidant production in pathology and normal physiology is limited. Calcium-independent phospholipases (iPLA2s) belong to a family of enzymes known for their involvement in cellular signaling through the production of free fatty acids and lysophospholipids. Our primary focus was on the release of fatty acids and their role in proton transport across the mitochondrial membrane. We confirmed that this proton transport increases the rate of respiration, leading to a decrease in oxidant production and thereby protecting cells from oxidative stress. Additionally, the antioxidant and anti-inflammatory role of iPLA2γ was also confirmed in vivo using a mouse model.
Through comprehensive lipidomic analyses, we identified a characteristic cleavage pattern associated with redox-activated iPLA2γ in the brain. Our data indicate an increase in the relative concentrations of specific fatty acids, such as docosahexaenoic acid, arachidonic acid, and stearic acid. This change was inhibited by a selective iPLA2γ inhibitor. Furthermore, we observed a significant decrease in several high molecular weight cardiolipin (CL) species, along with an accumulation of low molecular weight CL species in the brain mitochondria of iPLA2γ knockout mice. These findings suggest that phospholipase iPLA2γ facilitates the conversion of lower molecular weight, saturated cardiolipins into higher molecular weight, polyunsaturated fatty acid-rich cardiolipins, a characteristic profile of brain tissue.
Collectively, these results highlight the essential role of redox-activated iPLA2γ in redox signaling, leading to: (i) a subsequent attenuation of oxidant production, and (ii) post-synthetic remodeling of immature cardiolipins.
The study was supported by grants of the Czech Ministry of Education (MŠMT), LTA USA 17174.
Publications
Nahorniak; Mykhailo - Horák; Daniel - Šlouf; Miroslav - Steinhart; Miloš - Shapoval; Oleksandr - Engstová; Hana - Ježek; Petr Lanthanide-based UCNPs: toxicity evaluation and interaction of ultrasmall core vs. core-shell nanoparticles with cells. Materials Advances. 2025; 6(19); 6907-6918.
IF = 4.7
Shapoval; Oleksandr - Engstová; Hana - Šlouf; Miroslav - Kočková; Olga - Dlasková; Andrea - Jabůrek; Martin - Halili; A. - Mozheitova; Olexandra - Jirák; D. - Ježek; Petr - Horák; Daniel Liraglutide-conjugated poly(methyl vinyl ether-alt-maleic acid)-coated core-shell upconversion nanoparticles for theranostics of diabetes. ACS Applied Materials and Interfaces. 2025; 17(30); 42863-42876.
IF = 8.2
Rogulska; Olena - Vavřínová; E. - Vacková; Irena - Havelková; Jarmila - Gotvaldová; Klára - Abaffy; P. - Kubinová; Š. - Šíma; M. - Rössner ml.; P. - Bačáková; Lucie - Jendelová; P. - Smolková; Katarína - Petrenko; Yuriy The role of cytokine licensing in shaping the therapeutic potential of wharton’s jelly MSCs: metabolic shift towards immunomodulation at the expense of differentiation. Stem Cell Research & Therapy. 2025; 16(1); 199.
IF = 7.3