Laboratory of Bioenergetics
Content of this page
About the Laboratory
We study the physiology of mitochondria, cell organelles responsible for most of the energy production on the molecular level. We use both animal models and cells derived from patients harbouring various mitochondrial disorders. Our research is focused mainly on:
- Assembly of mitochondrial protein complexes and supercomplexes.
- Human diseases caused by mutations in assembly factors of these enzyme complexes.
- Development of protocols for diagnostics of mitochondrial diseases using patient-derived lymphocytes.
- Identification of new mitochondrial genes that play a causal role in the metabolic syndrome and heart failure.
Projects
Achievements
TMEM 70 disease
Animal models of human mitochondrial diseases enable detailed insight into pathogenic mechanisms, from molecular to organismal levels. More
Mitochondria dysfunction and Fanconi syndrome
Inborn disorders of energy provision by mitochondrial respiratory chain are the primary cause of numerous serious diseases, ranging from most severe encephalo-cardio-myopathies manifesting early after birth to various tissues-specific and milder disorders affecting mainly adults. More
Our picture among winners of a photo contest "Věda fotogenická"
Fourth year of the scienticic photography contest means success for our young guns.
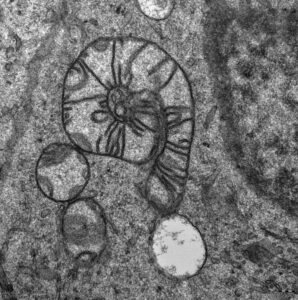
MitoSnail
Auttors: Lukáš Alán (IPHYS) and Marie Rodinová (CUNI)
Our new publication: High resistance to mitochondrial mutations
A developing organism requires high amounts of energy in the form of ATP. In higher eukaryotes, and thus in humans, more than 90 % of ATP is produced in mitochondria, a key organelle of the cellular catabolism. It is therefore not surprising that mitochondrial defects belong to the most frequent causes of metabolic diseases in children.
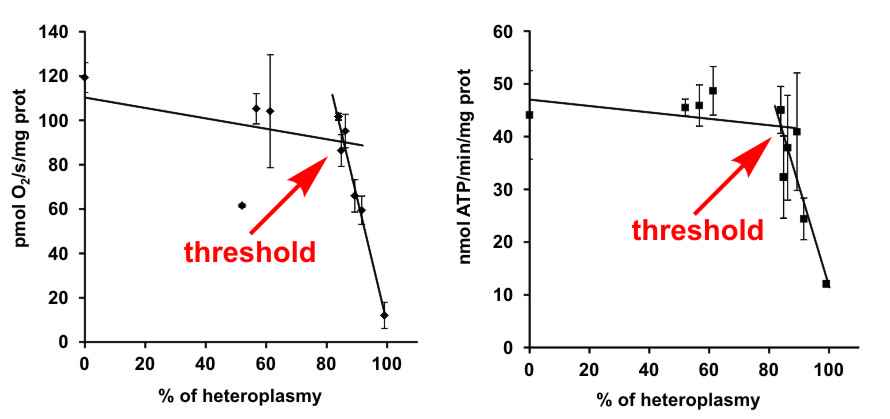
ATP synthase and other enzymes of the oxidative phosphorylation system share one unique trait, namely the dual genetic origin of their subunits, which are encoded by DNA from the nucleus and also by DNA in mitochondria (mtDNA). Both nuclear and mitochondrial DNA mutations may be manifested as hereditary diseases. Clinical symptoms of mtDNA patients usually vary from mild muscle dysfunctions to very severe heart and brain defects. Many different symptoms can be found also in patients with the same mutation, even in the siblings where the nuclear background has a minimal influence. The main factor affecting the disease severity is the so-called heteroplasmy that expresses the percentage of mutated mtDNA in respect to the healthy, wild-type mtDNA. This year, we published a study performed on the model of a rare mutation in the MT-ATP6 gene, leading to a defect of two oxidative phosphorylation enzymes: cytochrome c oxidase and ATP synthase. Although these are key enzymes for ATP production in the mammalian cell, we showed that a high threshold level of mutation load above 90 % of heteroplasmy is necessary to be reached for the biochemical manifestation of the mutation and thus for the development of severe symptoms. The aim of the study was to shed light on the relationship between the mutation load (level of heteroplasmy) and its clinical manifestation. In this specific case, the severity of symptoms is directly linked with the residual amount of the respective enzyme subunits, and the threshold originates from the gene/protein level.
Hejzlarová K, Kaplanová V, Nůsková H, et al. Alteration of structure and function of ATP synthase and cytochrome c oxidase by lack of Fo-a and Cox3 subunits caused by mitochondrial DNA 9205delTA mutation. Biochem. J. 2015;466:601–611.
Our papers in 2014
A brief list of what we published in 2014:
ATP SYNTHASE
Kratochvílová H, Hejzlarová K, Vrbacký M, et al. Mitochondrial membrane assembly of TMEM70 protein. Mitochondrion. 2014;15:1-9.
Hejzlarová K, Mráček T, Vrbacký M, et al. Nuclear genetic defects of mitochondrial ATP synthase. Physiol Res. 2014;63 Suppl 1:S57-S71.
SPONTANEOUSLY HYPERTENSIVE RAT
Trnovská J, Šilhavý J, Zídek V, et al. Gender-related effects on substrate utilization and metabolic adaptation in hairless spontaneously hypertensive rat. Physiol Res. 2015;64(1):51-60.
Houštěk J, Vrbacký M, Hejzlarová K, et al. Effects of mtDNA in SHR-mtF344 versus SHR conplastic strains on reduced OXPHOS enzyme levels, insulin resistance, cardiac hypertrophy, and systolic dysfunction. Physiol Genomics. 2014;46(18):671-678.
MITOCHONDRIAL ROS PRODUCTION
Shabalina IG, Vrbacký M, Pecinová A, et al. ROS production in brown adipose tissue mitochondria: The question of UCP1-dependence. Biochim Biophys Acta – Bioenerg. 2014;1837(12):2017-2030.
ROS modulation by membrane potential
One important paradigm of mitochondrial ROS production modulation states that high levels of mitochondrial membrane potential (ΔΨm) increase ROS generation in the respiratory chain. In a common project with the laboratory of Prof Cannon (The Wenner-Gren Institute, Stockholm, Sweden), we addressed this hypothesis on a model of brown adipose tissue (BAT) mitochondria, where ΔΨm is dissipated by the action of uncoupling protein 1 (UCP1). We compared the ROS production in BAT mitochondria isolated from wild-type mice or from UCP1−/− mice (with a high membrane potential) and found only ROS production supported by exogenously added succinate was affected by the presence of active UCP1. ROS production supported by any other tested substrate (including endogenously generated succinate) was not affected by ΔΨm. This indicates that UCP1 is not involved in the control of ROS production in BAT mitochondria, possibly indicating that also under other situations membrane depolarisation would not decrease physiologically relevant ROS production (Shabalina et al., Biochim Biophys Acta 1837: 2017, 2014)
Organization of respirometric workshop
The Faculty of Science, Charles University in Prague organized an international respirometric workshop (May 28–29, 2013). More
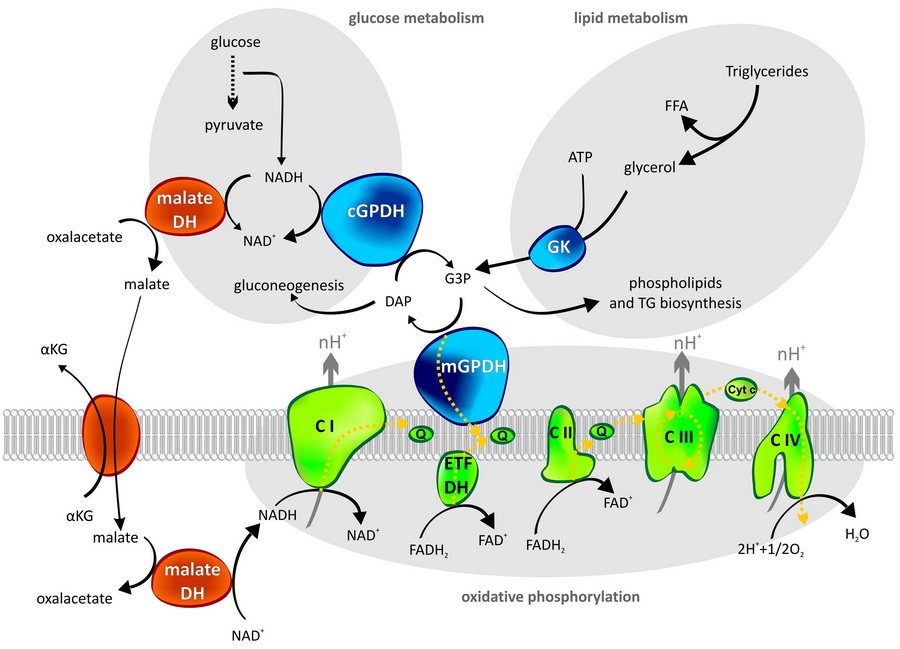
Our new paper: Mitochondrial glycerol-3-phosphate dehydrogenase and oxygen radicals
There is no fire without smoke. Similarly, cell respiration does not work perfectly and its by-products – reactive oxygen species (ROS), also known as free oxygen radicals – play a negative role in the development of many diseases as well as in aging.
We don’t know the detailed mechanism of ROS production for the majority of enzymes that have been shown to generate ROS. However, a known mechanism of this process is an essential prerequisite for searching for potential therapeutic compounds. We have recently shed light on the mechanism of ROS production by one of these enzymes – mitochondrial glycerol-3-phosphate dehydrogenase. You can read the paper in BBA Bioenergetics.
Tomáš Mráček, Eliška Holzerová, Zdeněk Drahota, Nikola Kovářová, Marek Vrbacký, Pavel Ješina, Josef Houštěk: ROS generation and multiple forms of mammalian mitochondrial glycerol-3-phosphate dehydrogenase
Mitochondrial glycerol-3-phosphate dehydrogenase
Is an important enzyme at the crossroads of glucose metabolism, lipid metabolism and mitochondrial oxidative phosphorylation. It represents one of the pathways, how to transport reducing equivalents from cytosol into mitochondria. In this so called glycerophosphate shuttle mGPDH has its cytosolic partner – cGPDH. In many cell types, glycerophosphate shuttle is only secondary to the better known malate/aspartate shuttle, which also transfers reducing equivalents to mitochondria.
Publications
Čunátová; Kristýna - Vrbacký; Marek - Puertas-Frias; Guillermo - Alán; Lukáš - Vanišová; M. - Saucedo-Rodríguez; María José - Houštěk; Josef - Fernández-Vizarra; E. - Neužil; Jiří - Pecinová; Alena - Pecina; Petr - Mráček; Tomáš Mitochondrial translation is the primary determinant of secondary mitochondrial complex I deficiencies. iScience. 2024; 27(8); 110560.
IF = 4.6
Alánová; Petra - Alán; Lukáš - Opletalová; Barbora - Bohuslavová; Romana - Abaffy; Pavel - Matějková; Kateřina - Holzerová; Kristýna - Benák; Daniel - Kaludercic; N. - Menabo; R. - Di Lisa; F. - Ošťádal; Bohuslav - Kolář; František - Pavlínková; Gabriela HIF-1α limits myocardial infarction by promoting mitophagy in mouse hearts adapted to chronic hypoxia. Acta Physiologica. 2024; 240(9); e14202.
IF = 5.6
Novotná; Eliška - Miloševič; Mirko - Průková; Dana - Magalhaes-Novais; Silvia - Dvořáková; Šárka - Dmytruk; Kristina - Gemperle; J. - Zudová; Dagmar - Nickl; Tereza - Vrbacký; Marek - Rosel; D. - Filimonenko; Vlada - Procházka; Jan - Brabek; J. - Neužil; Jiří - Rohlenová; Kateřina - Rohlena; Jakub Mitochondrial HER2 stimulates respiration and promotes tumorigenicity. European Journal of Clinical Investigation. 2024; 54(6).
IF = 4.4
Sharma; P. - Maklashina; E. - Voehler; M. - Balintová; Soňa - Dvořáková; Šárka - Kraus; Michal - Váňová Hadrava; Kateřina - Nahácka; Zuzana - Zobalová; Renata - Boukalová; Štěpána - Čunátová; Kristýna - Mráček; Tomáš - Ghayee; H. K. - Pacak; K. - Rohlena; Jakub - Neužil; Jiří - Cecchini; G. - Iverson; T. M. Disordered-to-ordered transitions in assembly factors allow the complex II catalytic subunit to switch binding partners. Nature Communications. 2024; 15(1); 473.
IF = 14.7