Laboratory of Biomathematics
Content of this page
The Laboratory of Biomathematics is active in fields of Bioimaging and Image Analysis.
About the Laboratory
Bioimaging and Image Analysis
The laboratory conducts research into the 3D microanatomical aspects of physiological phenomena at mesoscopic, microscopic and ultrastructural level. It is engaged in a range of collaborative projects across the campus and beyond. If interested please contact us (jiri.janacek@fgu.cas.cz).
CURRENTLY FUNDED PROJECTS
- Diabetic structural changes in capillary network and connective tissue
- Quantification of isolated pancreatic islets
LONG-TERM PROJECTS
- Development of 3D image analysis and stereological methods including software (employed, e.g., in a volumetric analysis of Langerhans islets from human donors)
- Microscopic optical-contrasting (label-free) strategies to visualize living cells and tissues
OTHER PROJECTS (selection only)
- Effects of ionizing radiation on blood capillaries network
- Effects of elevated CO2 on the photosynthetic apparatus’ performance and ultrastructure
- Mechanisms governing the formation of extracellular matrix proteins (collagen and elastin)
IN-HOUSE IMAGING MODALITIES
- Laser scanning confocal microscopy
- Two-photon excitation microscopy
- Second harmonic generation (SHG) microscopy
- ‘White-light‘ laser and hybrid detectors (on Leica TCS SP8 WLL MP microscope)
- FRAP recording, FRET analysis, FLIM/PLIM recording including λ2 scans
- Optical projection tomography (OPT)
- Selective plane illumination (light sheet) microscopy (SPIM)
- Phase-, modulation- and interference contrast microscopy
AVAILABLE SOFTWARE AND PROTOCOLS
- Software for 3D image analysis, stereology, spatial statistics and deconvolution (Hyughens)
- Optical/chemical clearing protocols for OPT and SPIM
OPEN ACCESS TO EQUIPMENT
The group is part of the Czech BioImaging infrastructure [1] supporting open access to most of its microscopes (listed above), and implicitly also to its expertise. The portfolio includes, among other imaging equipment located at other departments, a Nipkow spinning-disk (“Carv II“) confocal microscope [2].
[1] http://www.czech-bioimaging.cz/
[2] https://fgu.cas.cz/en/research-and-laboratories/research-departments/laboratory-of-biomathematics/
Projects
Achievements
Calmodulin and protein S100A1 bind in distal N- terminus of TRPM4 channel
It was identified new binding sites for the calmodulin (CaM) and S100A1, located in the very distal part of the TRPM4 N-terminus.
The TRPs channels N-and C- tails contain a number of conserved epitopes that specifically bind the intracellular modulators. It was identified new binding sites for the calmodulin (CaM) and S100A1, located in the very distal part of the TRPM4 N-terminus. We have used chemically synthesised peptides of the TRPM4, mimicking the binding epitopes, along with fluorescence methods to determine and specify CaM- and S100A1- binding sites. It was recognized that the ligands binding epitopes at the TRPM4 N-terminus overlap, but the interacting mechanism of both complexes is probably different. The molecular models supported by data from the fluorescence method confirmed that the complexes formations are mediated by the positively charged (R139, R140, R144) and hydrophobic (L134, L138, V143) residues present at the TRPM4 N-terminus binding epitopes. The data suggest that the molecular complexes of TRPM4/CaM and TRPM4/ S100A1 would lead to the modulation of the channel functions.
It was also investigated in silico whether TRPM4_CT can bind either Calmodulin (CaM) or S100A1. In canonical Ca2+-CaM peptide complexes, the peptide is anchored through interactions of two large hydrophobic residues to one of each Ca2+-CaM lobe. The N-terminal anchor forms extensive hydrophobic contacts with the surrounding residues of F92, L105, M124 and M144, lining a deep hydrophrobic pocket in the Ca2+-CaM C-lobe. These four residues form the tetrad. Similarly, there is tetrad in the Ca2+-CaM N-lobe formed by F19, L32, M51 and M71. The relative sequence position of the anchor residues defines the binding mode of Ca2+-CaM-peptide complexes. We searched the PDB and selected representative structures representing various canonical binding motifs (1-5-10, 1-8-14, 1-10, 1-14, etc.). The TRPM4_CT peptide contains several 1-10 motifs and thus it is possible to model its binding to Ca2+-CaM. However, in the context of the entire TRPM4, it has to be taken into account that the hydrophobic amino acids from the N-terminus of TRPM4_CT, are in close contacts with the sensor domain formed by helices S1-S4. Therefore, it can not be ruled out that the TRP re-entrant (TRPM4_CT) is bound to calmodulin by only one CaM lobe. It opens the possibility that CaM could stimulate the oligomerization of TRP ion channels. Alternatively, CaM (and S100A1) could stimulate dislocation of the TRP re-entrant segment from the S1-S4 sensor. Then, CaM and S100A1 comparing to PIP2 would appear to have exactly the opposite effects on gating of TRPM channels.
International poster award (Innsbruck 2016)
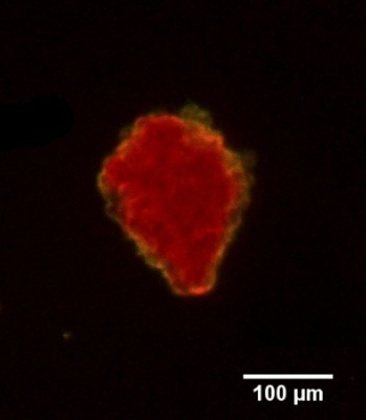
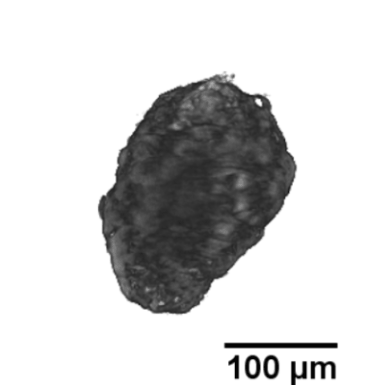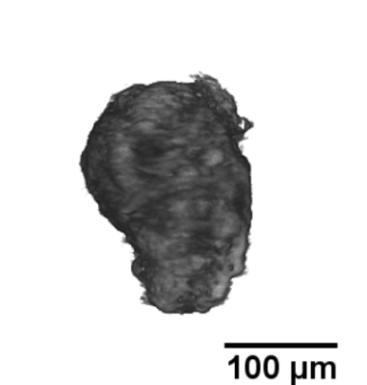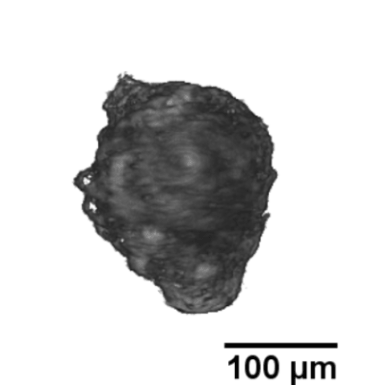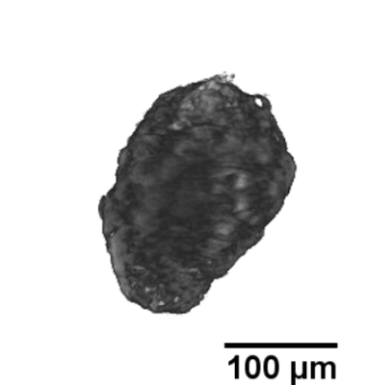
Langerhans islets’ volume measurements using optical projection tomography and the Fakir method
The study is part of a contribution “Real volumes of isolated human islets“ by David Habart from the Institute of Clinical & Experimental Medicine (IKEM), as carried out in collaboration with two faculties of the Czech Technical University, and Faculty of Mathematics and Physics, Charles University. The results were presented at the 6th European Pancreas and Islet Transplant Association (EPITA) & 35th Artificial Insulin Delivery, Pancreas and Islet Tranplantation (AIDPIT) Workshop (2016), and received the AIDPIT & EPITA poster award. The AIDPIT is one of Study Groups of the European Association for the Study of Diabetes. Among several procedures, our Fakir method has been chosen as a standard one to evaluate the islets’ volumes.
http://www.esot.org/events-education/events/3571/posters/1173
Sexual dimorphism in pheasant brains (Scientific Reports 2015)
Magnetic resonance and computed tomography study of avian brain compartments’ volumes measured by our Fakir probe revealed sexual dimorpism in pheasant brains.
A new precise volume measurement method is based upon coupled magnetic resonance (MR) imaging, computed tomography (CT) . Our approach utilizes a novel interactive Fakir probe cross-referenced with an automated CT protocol to efficiently generate total volumes and surface areas of the brain tissue and endoneurocranial space, as well as the discrete cephalic compartments. We also complemented our procedures by using sodium polytungstate (SPT) as a contrast agent. This greatly enhanced CT applications but did not degrade MR quality and is therefore practical for virtual brain tissue reconstructions employing multiple imaging modalities. To demonstrate our technique, we visualized sex-based brain size differentiation in a sample set of Ring-necked pheasants (Phasianus colchicus). This revealed no significant variance in relative volume or surface areas of the primary brain regions. Rather, a trend towards isometric enlargement of the total brain and endoneurocranial space was evidenced in males versus females, thus advocating a non-differential sexually dimorphic pattern of brain size increase amongst these facultatively flying birds.
Jirák D., Janáček J. and Kear B.P. (2015): A combined MR and CT study for precise quantitative analysis of the avian brain. Scientific Reports 5: 16002
Invited review on confocal stereology (Cell & Tissue Research 2015)
A review article on confocal stereology comprising innovative methods invented at the Department of Biomathematics
Confocal stereology represents a contemporary approach to evaluation of microscopic structures by using a combination of stereological methods and confocal microscopy. 3-D images acquired by confocal microscopy can be used for estimation of geometrical characteristics of microscopic structures by stereological methods based on evaluation of optical sections within a thick slice and using computer-generated virtual test probes. Such methods can be used for estimating volume, number, surface area and length using relevant spatial probes, which are generated by specific software. The interactions of the probes with the structure under study are interactively evaluated. Overview of the methods of confocal stereology developed during past 30 years is presented. Their advantages and pitfalls in comparison with other methods for measurement of geometrical characteristics of microscopic structure are discussed. Our Fakir and Slicer methods as our implementations of Disector and other methods were sucessfully applied in morphological studies of various organs.
Kubínová L. and Janáček J. (2015) Confocal stereology: an efficient tool for measurement of microscopic structures. Cell & Tissue Research 360: 13–28
Capillary length estimation (Microscopy & Microanalysis 2013)
Three types of capillary length estimation methods were compared:
- Stereological methods based on a computer generation of isotropic uniform random virtual test probes in 3D, either in the form of spatial grids of virtual “slicer” planes, or spherical probes.
- Automatic method employing a digital version of the Crofton relations using the Euler characteristic of planar sections of the binary image.
- Interactive “tracer” method for length measurement based on a manual delineation in 3D of the axes of capillary segments. The presented methods were compared in terms of their practical applicability, efficiency and precision.
Response of forest tree species to elevated carbon dioxide concentration: Chloroplast ultrastructure
On the level of chloroplast ultrastructure, we investigate the impact of elevated carbon dioxide concentration and irradiance on structure and performance of forest tree photosynthetic apparatus.
We use transmission electron microscopy, stereology and dual-axis electron tomography as tools to investigate ultrastructural changes in chloroplasts. This work is part of the project that aims to contribute to elucidation of changes to mesophyll cell photosynthetic performance of Norway spruce and European beech at different hierarchical levels and mechanisms lying behind, and to the knowledge on carbon sequestration by temperate trees. Sample preparation Dr. Radochová, data acquisition Dr. Bílý (Parasitol. Inst., CAS), dual axis tomography reconstruction Dr. Michálek, 3D volume rendering Dr. Janáček.
3D visualization of beech chloroplast acquired via dual-axis electron tomography in which thylakoids are colored green, starch grains are colored yellow and lipid particles (plastoglobuli) are colored brown. Note a cytoplasm-filled pocket with mitochondria colored blue.
Image processing in microscopy (IEEE Trans. Med. Imaging 2013)
We proposed an algorithm for alignment of neighbouring physical slices with ruptures in microscopy, based on iterative minimization of the problem dual to the L1-TV functional using the method of subgradients.
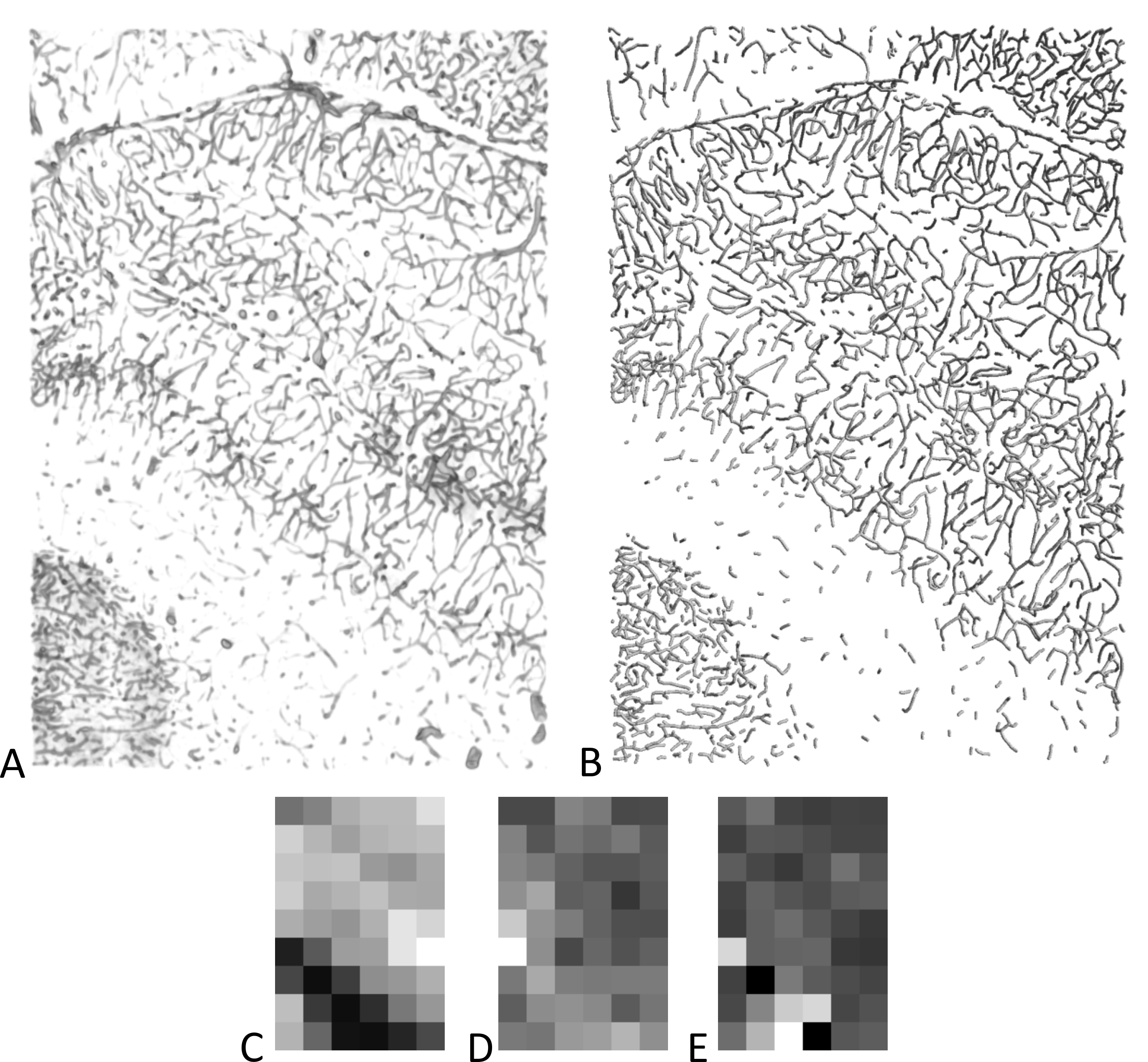
Quantitative measurement of capillaries
We study capillary bed using stereology and 3D image analysis.
The object of our study is an effect of the ionizing radiation on blood capillaries in various tissues and organs. We collaborate with team of Dr. Mao (Department of Radiation Medicine, Loma Linda University, CA, USA) on project focused on proton therapy.
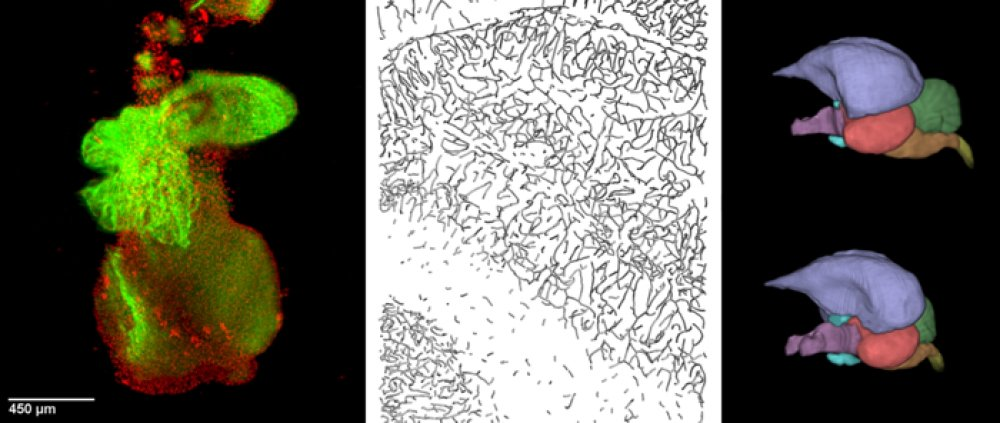
A) Volume rendering of composite images (70 confocal stacks in 10 rows and 7 columns, 1762×2519×80 µm) and B) surface rendering of skeletonized capillaries as cylinders. Images were obtained by a confocal microscope from perfusion stained samples of rat brain. C–E) Local measurements of the data in figure A, B. The image was split into 6×9 tiles before measurement and the value was represented by the gray level. Minimal value is represented by black. C) Length density, maximal value is 695 mm-2, D) anisotropy characteristics (0 to 1), maximal value is 0.74, E) average branch length, maximal value is 0.7 mm.
Publications
Habart; D. - Koza; A. - Leontovyč; I. - Kosinová; L. - Berková; Z. - Kříž; J. - Zacharovová; K. - Brinkhof; B. - Cornelissen; D. J. - Magrane; N. - Bittenglová; K. - Čapek; Martin - Valečka; J. - Habartová; Alena - Saudek; F. IsletSwipe; a mobile platform for expert opinion exchange on islet graft images. Islets. 2023; 15(1)); 2189873.
IF = 2.2
Olejníčková; Veronika - Hamor; P. U. - Janáček; Jiří - Bartoš; M. - Zábrodská; E. - Šaňková; B. - Kvasilová; A. - Kolesová; Hana - Sedmera; David Development of ventricular trabeculae affects electrical conduction in the early endothermic heart. Developmental Dynamics. 2024; 253(1); 78-90.
IF = 2.5
Gregorovičová; M. - Bartoš; M. - Jensen; B. - Janáček; Jiří - Minne; B. - Moravec; J. - Sedmera; David Anguimorpha as a model group for studying the comparative heart morphology among Lepidosauria: Evolutionary window on the ventricular septation. Ecology and Evolution. 2022; 12(11)); e9476.
IF = 2.6