Laboratory of Pancreatic Islet Research
Content of this page

About the Laboratory
The ability of β-cells to function as glucose sensors is crucial for maintaining glucose homeostasis throughout life. A decline in β-cell function and quantity is a key indicator of diabetes onset and progression. The activity of β-cells is closely interlinked with other endocrine cells in the pancreatic islets. While insulin was discovered over a century ago (in 1922) and its main secretion pathways are well understood, much remains unknown about the molecular mechanisms within β-cells and pancreatic islets. This knowledge is essential for developing effective strategies to prevent and treat diabetes, one of the most pressing global health challenges.
Our laboratory focuses on exploring the role of redox homeostasis in insulin secretion under both normal physiological conditions and in the context of pathological pro-oxidative signaling, which contributes to diabetes development. We employ a range of models, from cell cultures and isolated mouse islets of Langerhans to whole-animal studies in mice, to investigate pancreatic β-cell and islet biology. By combining innovative experimental approaches with advanced omics technologies, we aim to uncover new insights that can drive the prevention and treatment of diabetes.

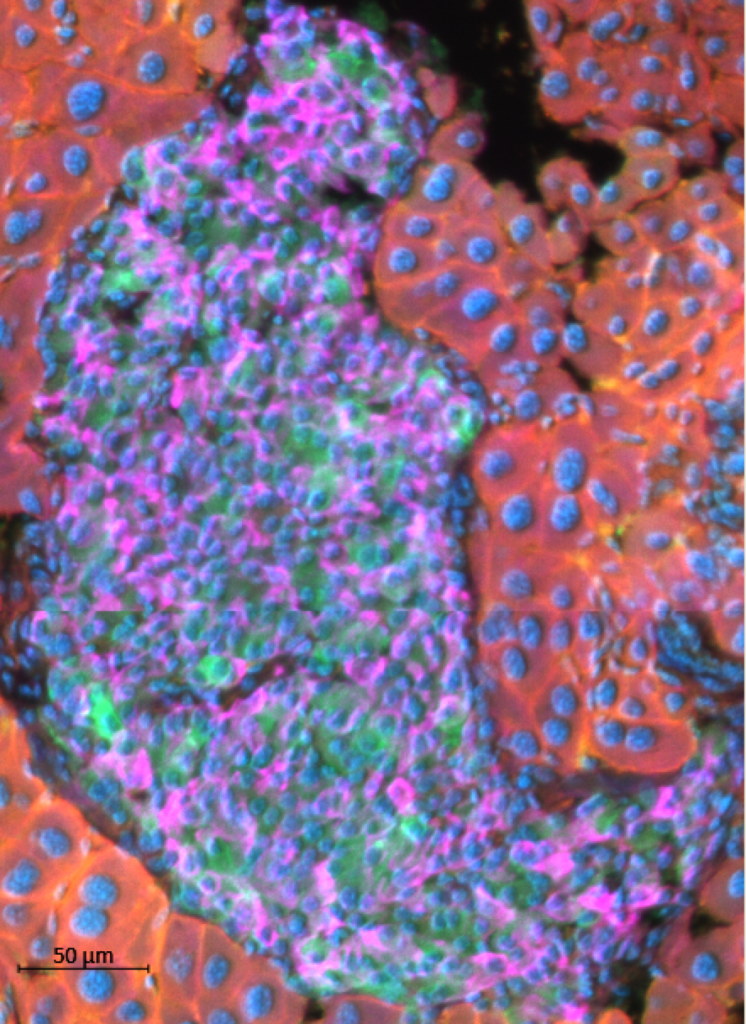
Fluorescent insulin injected into a mouse pancreatic islet
Projects
Achievements
We have elucidated the role of NADPH oxidase 4 (NOX4) in pancreatic beta-cells
We successfully identified the role of NADPH oxidase 4 (NOX4) in regulating glucose-stimulated insulin secretion (Plecitá-Hlavatá, L., 2020, Diabetes). Additionally, we have uncovered key protein targets involved in this redox signaling pathway (Holendova, B., 2024, Metabolism). Our research also demonstrated that chronic NOX4 activity, triggered by metabolic stress such as prolonged overeating, leads to inflammasome activation and localized inflammation (Holendova, B., 2024, Obesity).
Publications
Li; M. - Plecitá-Hlavatá; Lydie - Dobrinskikh; E. - McKeon; B. A. - Gandjeva; A. - Riddle; S. - Laux; A. - Prasad; R. R. - Kumar; S. - Tuder; R. M. - Zhang; H. - Hu; Ch.-J. - Stenmark; K. R. SIRT3 Is a Critical Regulator of Mitochondrial Function of Fibroblasts in Pulmonary Hypertension. American Journal of Respiratory Cell and Molecular Biology. 2023; 69(5); 570-583.
IF = 5.9