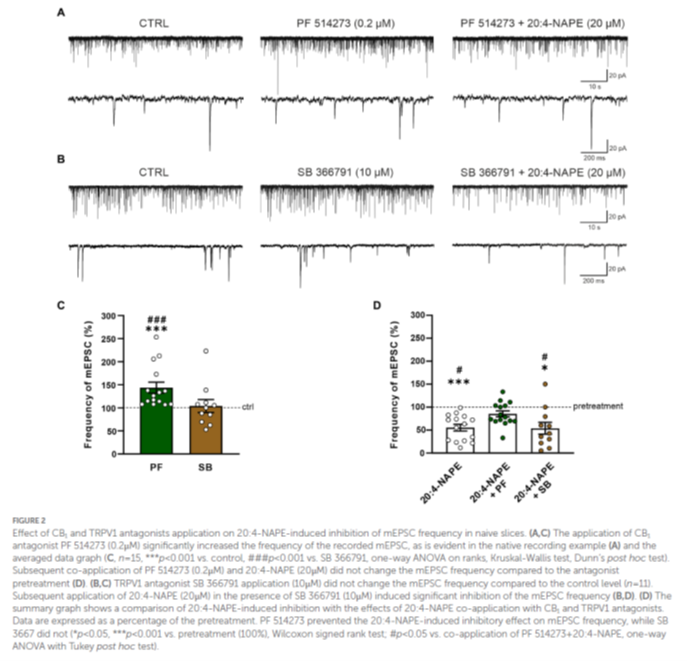
Our laboratory has recently published a study in Frontiers in Molecular Neuroscience that uncovers a novel mechanism by which the body’s own lipid messengers influence pain processing. The focus of the research was 20:4-NAPE, a naturally occurring lipid and precursor of the endocannabinoid anandamide, which is known to interact with both cannabinoid (CB1) and vanilloid (TRPV1) receptors. These receptors are essential for regulating pain signals in the spinal cord, yet their interplay during inflammation has remained poorly understood.
Using advanced patch-clamp electrophysiology in rat spinal cord slices, we demonstrated that 20:4-NAPE profoundly reduces synaptic activity between pain-transmitting neurons. Under normal conditions, this effect was mediated primarily through CB1 receptors. However, in the presence of peripheral inflammation, the mechanism strikingly shifted: the inhibitory action of 20:4-NAPE was no longer dependent on CB1, but instead relied on activation of TRPV1 receptors. This switch in receptor pathways highlights how inflammation dynamically reshapes the molecular environment of spinal synapses.
Our findings reveal that 20:4-NAPE is not just a passive precursor of anandamide, but an active regulator of pain signaling with context-dependent actions. By identifying the inflammation-driven switch from CB1 to TRPV1 signaling, we provide new insights into the complexity of endocannabinoid and endovanilloid systems in the spinal cord. This discovery has important implications for the development of next-generation pain therapies, particularly for chronic inflammatory conditions where conventional treatments are often insufficient.
This research underscores our laboratory’s commitment to advancing fundamental neuroscience while paving the way toward innovative therapeutic approaches for patients suffering from persistent pain.
Spicarova D, Nerandzic V, Muzik D, Pontearso M, Bhattacharyya A, Nagy I and Palecek J (2023) Inhibition of synaptic transmission by anandamide precursor 20:4-NAPE is mediated by TRPV1 receptors under inflammatory conditions. Front. Mol. Neurosci. 16:1188503. doi: 10.3389/fnmol.2023.1188503