Molecular mechanisms of TRPC5 ion channel activation and regulation
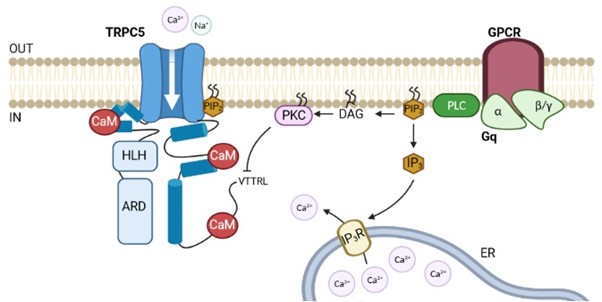
The human transient receptor potential canonical 5 (TRPC5) is a polymodal, calcium-permeable, non-selective cation channel mainly activated by G-proteins and their downstream signal transduction pathways, but also by noxious cold temperatures [1,2]. It is expressed in the central and peripheral nervous system and in other tissues such as kidney, synovium and odontoblasts. It has recently been shown that pharmacological and genetic inhibition of TRPC5 leads to relief of neuropathic and inflammatory pain, making TRPC5 an attractive target for pain management strategies.
Compared to TRPA1 and TRPV1, very little attention has been paid to TRPC5 research so far. This was partly due to the lack of specific agonists and inhibitors, and partly due to the lack of accurate structural data. However, both of these obstacles have been overcome in recent years, offering a unique opportunity to expand our knowledge of TRPC5 mechanisms of action.
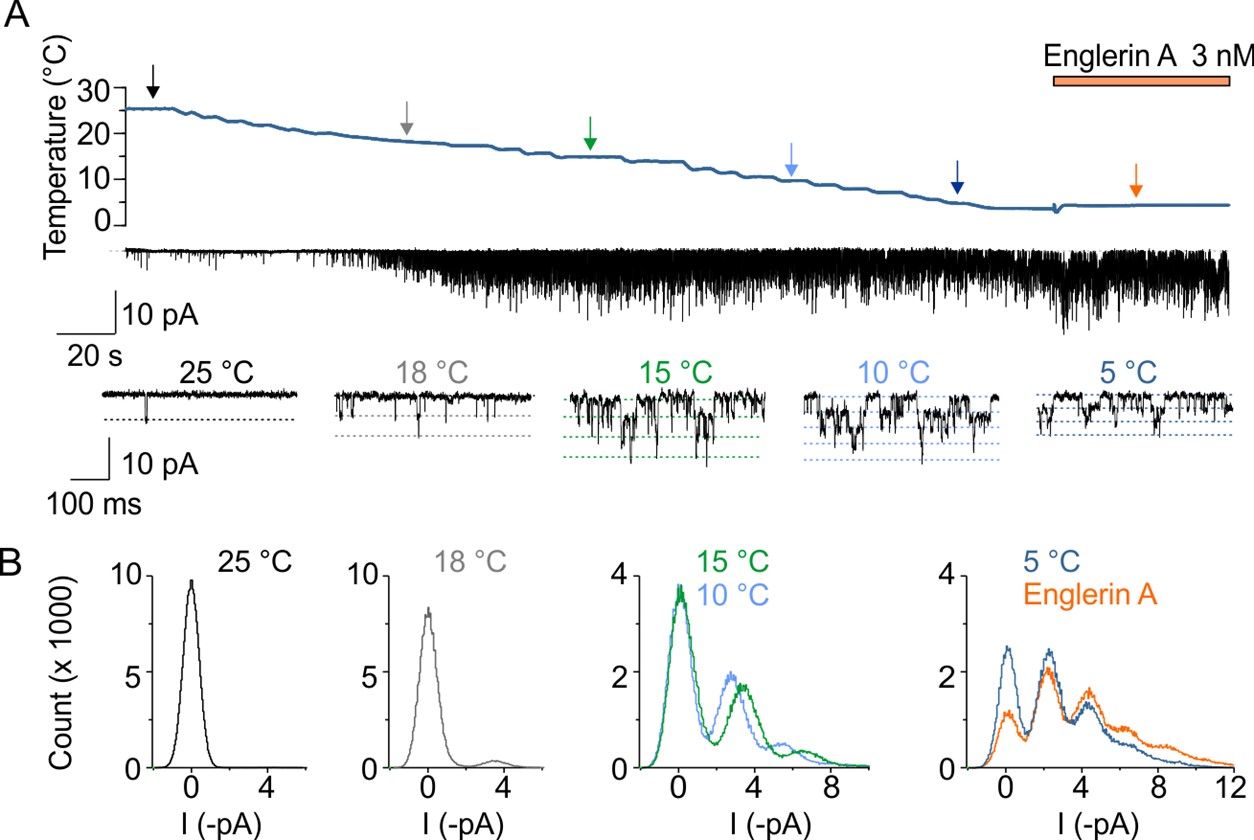
Our lab have extensive experience in characterizing ion channel temperature properties. These proved essential for our project aimed at determining the primary characteristics of TRPC5 as a cold-activated channel. We explored the cold sensitivity of human TRPC5 at the single-channel level using transiently transfected HEK293T cells. In the control extracellular solution, as the temperature decreased, the open probability increased robustly between 16 °C and 11 °C and reached saturation below 5-8 °C. Thermodynamic analysis revealed significant changes in enthalpy and entropy suggesting that substantial conformational changes accompany cold-induced gating. Our experiments confirmed that TRPC5 exhibits features of intrinsically cold-gated channel and furthermore showed that temperature-dependent gating of TRPC5 is critically regulated by intracellular regulatory pathways. [3]
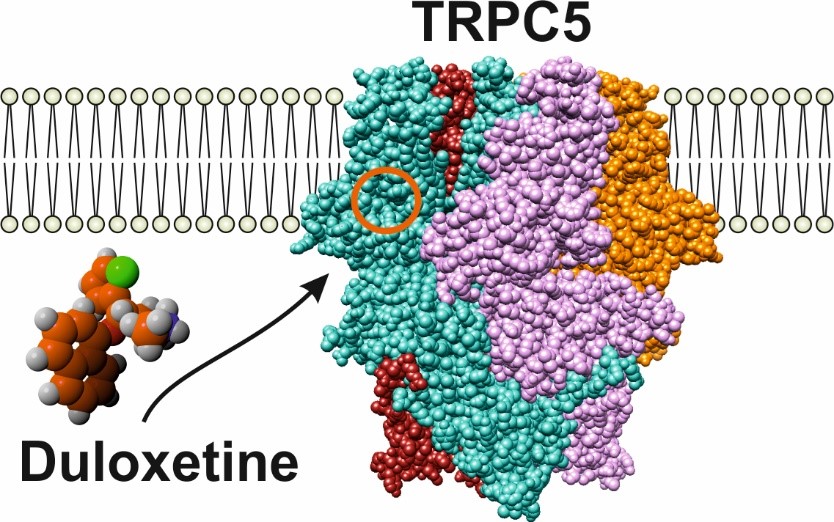
Despite great advances in medicine, treatment options for pain states associated with diabetes or chemotherapy-induced neuropathy are still limited. These types of neuropathies are accompanied by cold-induced pain that is very difficult to manage in clinical practice. In fact, the only drug that has successfully undergone clinical trials and demonstrated efficacy for this type of pain so far is duloxetine. This drug is superior to other antidepressants with a similar mechanism of action at managing painful neuropathies, but it is not known why. Interestingly, it has recently been shown that the cold-sensitive TRPC5 ion channel is expressed in human sensory neurons and that inhibition of its activity relieves persistent pain, including neuropathic cold pain.
In our study we asked whether the TRPC5 channel is modulated by duloxetine and may contribute to its analgesic effect. Our electrophysiological measurements showed that TRPC5 channel activity is strongly suppressed by duloxetine. We performed molecular docking and molecular dynamic simulations that identified a potential biding site for duloxetine. Subsequent point mutagenesis validated that the duloxetine molecule resides in a well-known biding pocket on the intracellular side of the TRPC5 transmembrane domain. Slight manipulation of the shape and electrostatic of the binding pocket environment (replacing the amino acid glutamate 418 with alanine) caused a complete loss of the duloxetine effect on voltage-evoked TRPC5 activity. Our results suggest that TRPC5 is a previously unrecognised target for a commonly used, highly effective drug against severe forms of pain. Furthermore, the finding that this TRPC5 inhibitor is widely used and well tolerated provides a scaffold for new pain treatment strategies. [4]
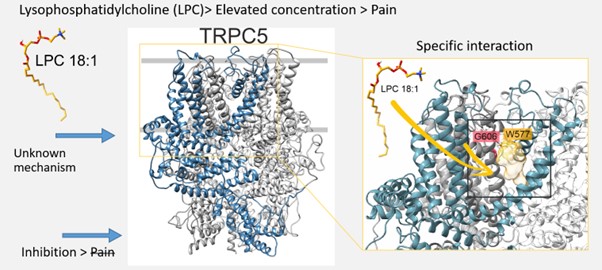
Lysophosphatidylcholine (LPC) has emerged as a pivotal endogenous factor in the modulation of pain, particularly in chronic pain states. Research indicates that LPC levels are significantly elevated in conditions such as fibromyalgia and osteoarthritis, correlating with heightened pain experiences in affected individuals. TRPC5 has been identified as a key contributor to mechanical hypersensitivity associated with various inflammatory and neuropathic pain models, where LPC acts as an endogenous agonist that directly activates the channel.
In our investigations [5], we focused on the impact of LPC on TRPC5 gating mechanisms, revealing that LPC-induced activation is intricately modulated by xanthine ligands and depolarizing voltages. This process involves conserved residues within the lateral fenestration of the pore domain of TRPC5, which are crucial for its response to LPC. These findings not only enhance our understanding of TRPC5’s role in pain signaling but also suggest potential therapeutic avenues for targeting TRPC5 in conditions characterized by elevated LPC levels and associated chronic pain.
[1] Ptakova, A., & Vlachova, V. (2024). Thermosensing ability of TRPC5: current knowledge and unsettled questions. The Journal of Physiological Sciences, 74(1), 50. https://doi.org/10.1186/s12576-024-00942-3
[2] Khare, P., Chand, J., Ptakova, A., Liguori, R., Ferrazzi, F., Bishnoi, M., Vlachova, V. and Zimmermann, K., (2024). The TRPC5 receptor as pharmacological target for pain and metabolic disease. Pharmacology & Therapeutics, p.108727. https://doi.org/10.1016/j.pharmthera.2024.108727
[3] Ptakova, A., Mitro, M., Zimova, L., & Vlachova, V. (2022). Cellular context determines primary characteristics of human TRPC5 as a cold‐activated channel. Journal of Cellular Physiology, 237(9), 3614-3626. https://doi.org/10.1002/jcp.30821
[4] Zimova, L., Ptakova, A., Mitro, M., Krusek, J., & Vlachova, V. (2022). Activity dependent inhibition of TRPC1/4/5 channels by duloxetine involves voltage sensor-like domain. Biomedicine & Pharmacotherapy, 152, 113262. https://doi.org/10.1016/j.biopha.2022.113262
[5] Ptakova, A., Zimova, L., Barvik, I., Bon, R. S., & Vlachova, V. (2024). Functional determinants of lysophospholipid-and voltage-dependent regulation of TRPC5 channel. Cellular and Molecular Life Sciences, 81(1), 374. https://doi.org/10.1007/s00018-024-05417-7