Biological Controls
Content of this page

About the department
Department of Biological Controls provides fee-based services for nonclinical safety testing on small laboratory animals. OBK serves as registered Test Facility in the Czech Republic. OBK uses the validated state of the art equipment, established metrology program. OBK makes use of experimental stables for small animals, rooms are air conditioned, humidity is controlled, environmental parameters are continuously collected to demonstrate compliance.There are the skilled study directors, pathologists and other support personnel staff.
OBK is a key member of the Center for Preclinical Testing.
CONTACT
tel. +420 241 062 806, email: cptmail@fgu.cas.cz
OBK Services
2.6. 2021
- GLP and non-GLP in – vivo studies to support preclinical development and CTA/MA processes
- Custom tailored or standard safety testing programs
- Test Items: Human and veterinary medicinal products, food supplements, medical devices
- Test systems: mouse, rat, guinea pig, rabbit (non rodents in collaboration with Pigmod centre)
- Administration routes: common administration routes (oral, intravenous, intradermal, intramuscular, intraperitoneal, subcutaneous)
- Test Guidelines: ICH, OECD, EMA, ISO
- Acute toxicity, MTD studies (GLP/non-GLP)
- Repeated dose, DRF studies (GLP/non-GLP)
- PK and ADME (Bio-distribution) studies (GLP/non-GLP)
- Proof of Concept studies (Pharmacology)
- In vivo biocompatibility studies (for medical devices)
- Hematological testing of blood / plasma / serum from test animals -TECHNICAL DETAILS
- Gross pathology evaluation
- Histopathology
- Consultancy on design of preclinical activities
Typical non-clinical safety study workflow:
- Drafting and approval of Study plan
- Animals: intake, entry veterinary examination, randomization, acclimation
- Test Item (TI) receipt, Identification, sampling
- Takeover of Test Item and proper storage
- Preparation of application form + administration to animals
- Daily observation, weighing, blood sampling of test animals
- Gross pathology observations
- Histopathology
- Final Report
Before starting detailed discussion on study design, please give us information on your intention, basic study design (animal species, number of animals/groups, administration route, information on item to be tested) and any other information that can help us understand your needs. Put all this information into Message window below and send it to us. We will respond promptly.
Production of whole blood, plasma, serum and rabbit complement, guinea-pig complement
Whole blood
- Blood sampling with anticoagulant
Plasma
- Blood sampling with anticoagulant,
- Centrifugation,
- Supernatant liquid sampling = plasma
- Freezing
Serum
- Blood sampling without any anticoagulant,
- Centrifugation,
- Supernatant liquid sampling = serum
- Freezing
Complement
- Preparation of complement
- The Rabbit complement is supplied frozen, package of 1ml or 2ml
Immunization of animals and hyperimmune sera preparation
Download:
CERTIFICATE OF GOOD LABORATORY PRACTICE (GLP) for non-clinical testing of Pharmaceuticals
Hematological testing of blood / plasma / serum from test animals
Technical details of hematological testing
Animal species: mouse, rat, dog, cat, horse, ferret
Measured parameters: WBC, RBC, HGB, HCT, MCV, MCH, MCHC, RDW, PLT, MPV, LYM, MON, GRA, EOS
Animal species: rabbit, pig, sheep, cattle
Measured parameters: WBC, RBC, HGB, HCT, MCV, MCH, MCHC, PLT
Minimum sample volume: 50 μl EDTA blood
Blood measurement should be done within 4 hours after blood collection
Technical specifications
Method: RBC, WBC and PLTS- electrical impedance, hemoglobin – spectral photometry
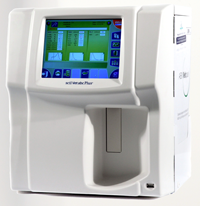
scil Vet abc Plus+: Analytical instrument to determine hematological parameters from whole blood
Coagulation parameters testing from blood plasma of test animals
Technical details
Animal species: rat
Measured parameters: APTT, PT
Minimum sample volume: 60 μl blood citrate plasma
Rayto RT-2204 C Analytical instrument to determine coagulation parameters from blood plasma
Citrate plasma preparation
Venous blood should be carefully collected into a tube treated by sodium citrate solution. Attention should be paid to maintain of ratio of 9 parts blood to 1 part citrate. Tubes with blood should be mixed well and centrifuged (15 minutes, 2500 g, 20 °C) as soon as possible after blood collection. Plasma samples should be maintained at room temperature and should be tested within 4 hours by PT test and within 8 hours by APTT test.
Untested residue of citrate plasma should be processed to platelet-free citrate plasma by second centrifugation (30 minutes, 3000 g, 20 °C). Separated platelet-free plasma should be immediately frozen at -80 °C for further possible testing.
Frozen platelet-free citrate plasma should be thawed at 37 °C for 15 minutes and then should be mixed well and centrifuged (5 minutes, 500 RPM, 20 °C). The plasma should be tested immediately after centrifugation.














