The findings of scientists from the Department of Molecular Neurobiology who work together with scientists from Harvard University, may contribute to the understanding of the pathological processes during aging of neurons and origin of Alzheimer’s disease.
Amino acids are linked by peptide bonds in the polypeptide chain forming the basis of proteins in two ways in terms of the spatial arrangement (conformation) of their side chains – cis or trans. These conformations of the peptide bonds can vary from one another, but the vast majority (99.9%) are in the more energetically favorable trans conformation, which ensures an even distribution of side chains. The exception is the amino acid proline, whose structure breaks the symmetry of the polypeptide chain, making it easier to shift between trans and cis conformations. Changes in trans-cis conformation affect the structure and function of the entire protein. To maintain the functional conformation of proteins, cells use enzymes (prolyl isomerases) that catalyze the shift of misfolded proteins at the proline site to their native conformation. One of the most important prolyl isomerases (Pin1) controls a large number of cellular processes through the regulation of the conformation of its substrates, and its deregulation has been associated with the development of tumors, Alzheimer’s disease, and other diseases. However, the function of Pin1 isomerase in healthy neurons has not yet been determined.
A team from the Department of Molecular Neurobiology, in collaboration with scientists from Harvard University, recently showed that an important function of Pin1 isomerase in neurons is to regulate the growth of axons (long neuronal processes) during nervous system development. Using mouse and fish models, the research team showed that the absence of Pin1 isomerase leads to defects in axon guidance during embryonic development. In aging neurons, Pin1 isomerase dysfunction (and associated conformational stress) is associated with the development of Alzheimer’s disease. Thus, the new data are not only important in terms of neurodevelopmental diseases, but may contribute to the understanding of the pathological processes induced by Pin1 isomerase deregulation during neuronal aging and the development of Alzheimer’s disease.
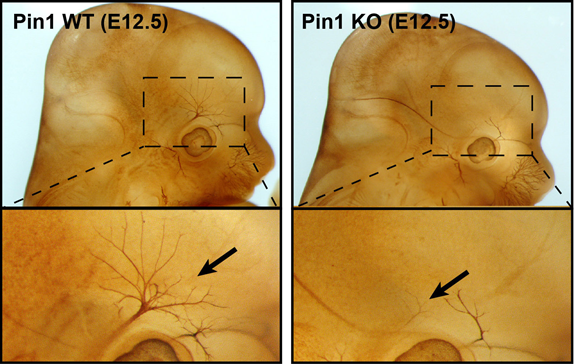
Changes in the growth of head nerve axons (nervus ophtalmicus) in Pin1 deficient embryos (Pin1 KO, age 12.5 days) compared to control embryos (Pin1 WT) (stained with antibodies to Neurofilament L subunit).
Balastik M, Zhou XZ, Alberich-Jorda M, Weissova R, Žiak J, Pazyra-Murphy MF, Cosker KE, Machonova O, Kozmikova I, Chen CH, Pastorino L, Asara JM, Cole A, Sutherland C, Segal RA, Lu KP. Prolyl Isomerase Pin1 Regulates Axon Guidance by Stabilizing CRMP2A Selectively in Distal Axons. Cell Rep. 2015 Oct 27;13(4):812-28.