Temperature-sensitive TRP ion channels are cellular molecular sensors involved in the transduction of sensory signals, pain perception (nociception) and the maintenance of ion homeostasis. Disturbances in their function are associated with many serious human diseases such as chronic pain, inflammation, cancer and various cardiovascular, neurological, respiratory, renal and metabolic disorders. The enormous hope of developing new drugs targeting these channels is greatly limited due to their intrinsic ability to be activated by stimuli of different modalities (polymodality), the mechanism of which has not yet been fully understood. An international team of scientists led by Prof. Peter M. Zygmund (Lund University, Malmö, Sweden), in collaboration with scientists from the Institute of Physiology of the Czech Academy of Sciences in Prague, has revealed two important mechanisms contributing to the polymodal regulation of TRP channels: The first study revealed the binding site and molecular nature of the binding of D9-tetrahydrocannabiorcol (a natural plant cannabinoid with no psychotropic effects), which strongly facilitates the activation of TRPV2, thereby affecting the transmission of painful stimuli. The second study identified two separate specific regions that confer heat (> 35 °C) and cold (< 15 °C) sensitivity to a different receptor, TRPA1, and demonstrated that the temperature sensitivity of this receptor is critically dependent on oxidative and reducing environments. The results make an important contribution to the understanding of the general molecular mechanisms of chemical and thermal activation of TRP family channels and will find application in the search for possible approaches to their regulation by drugs.
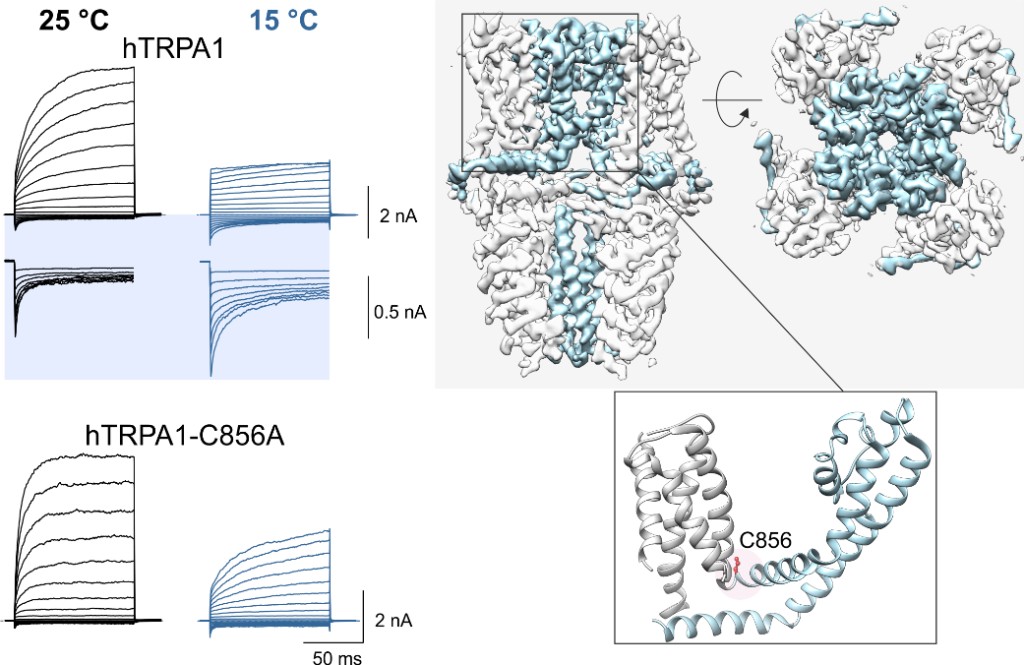
Left panel: effect of cold (15°C) on activation of the human TRPA1 wild-type ion channel and a mutant in which the major amino acid residue C856 responsible for regulation by oxidizing and reducing agents is substituted for alanine. The channels were activated by a series of voltage pulses (from -160 mV to +200 mV). Right panel: visualization of the TRPA1 channel structure obtained by cryoelectron microscopy (PDB: 6v9w) as viewed from the side and top. Dynamic regions of the channel responsible for cold activation are highlighted in light blue. The position of cysteine C856 is shown in the detail of the structure of one subunit below.
Moparthi, L. – Sinica, Viktor – Moparthi, V. K. – Kreir, M. – Vignane, T. – Filipovic, M. R. – Vlachová, Viktorie – Zygmunt, P. M.The human TRPA1 intrinsic cold and heat sensitivity involves separate channel structures beyond the N-ARD domain. Nature Communications. Roc. 13, c. 1 (2022), IF: 17.694 DOI
Zhang, L. – Simonsen, Ch. – Zímová, Lucie – Wang, K. – Moparthi, L. – Gaudet, R. – Ekoff, M. – Nilsson, G. – Hellmich, U. A. – Vlachová, Viktorie – Gourdon, P. – Zygmunt, P. M. Cannabinoid non-cannabidiol site modulation of TRPV2 structure and function. Nature Communications. Roc. 13, c. 1 (2022), IF: 17.694, rok: 2021 DOI