Laboratory of Biomaterials and Tissue Engineering

Content of this page
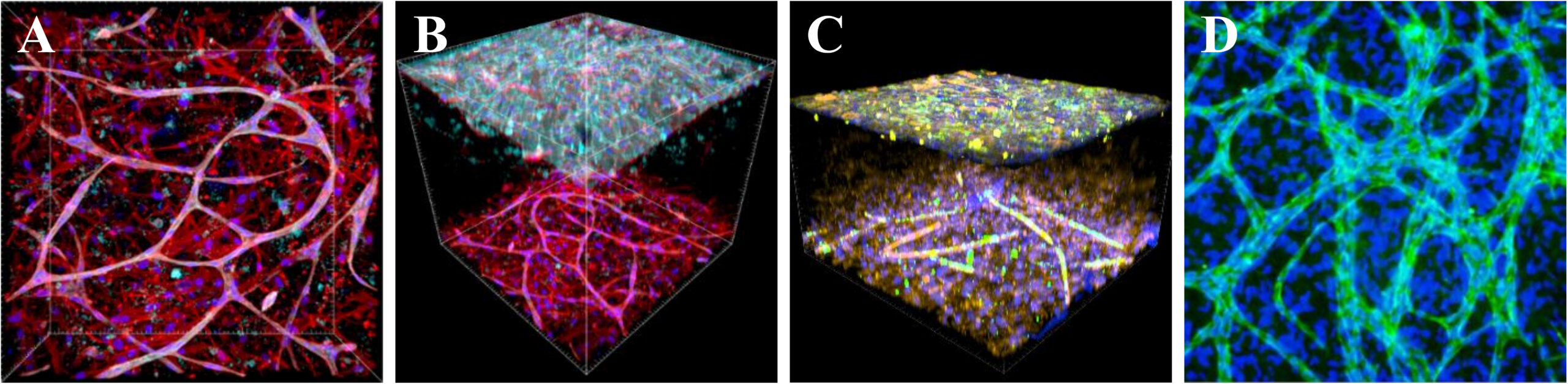
A versatile pre-vascularized construct for tissue engineering of the vascular wall, skin and bone. A: Mesenchymal stem cells and endothelial cells spontaneously form pre-capillaries in a hydrogel. This assembly is then the basis for the formation of the tunica intima and media complex of the vascular wall (B), the dermal-epidermal construct of the skin (C), and vascularized bone tissue (D).
About the Laboratory
Irreversible damage to essential tissues and organs, such as blood vessels, heart valves, bones, joints, cartilage, or skin, is a serious and common consequence of modern lifestyles. It relates to various metabolic disorders, as well as injuries resulting from traffic, sports, and work-related activities. Congenital defects, along with physiological wear and tear and the natural aging process, also contribute to this issue.
Our laboratory traditionally concentrates on three main areas: tissue engineering of blood vessels, skin, and connective tissues, including bone and cartilage. Within each of these areas, we focus on three key tasks:
(1) improving existing tissue replacements, especially by adding cellular and other biological components,
(2) developing completely new replacements using synthetic and natural biomaterials along with cells,
(3) creating three-dimensional (3D) tissue models in vitro for various physiological, pathophysiological, and pharmacological studies aimed at replacing laboratory animals in modern science, following the 3R principle. Examples include in vitro models of hypertrophic scars, Dupuytren’s contracture, and orthopedic defects such as clubfoot (Pes equinovarus).
Our subsidiary laboratory at BIOCEV in Vestec, near Prague (led by Dr. Yuriy Petrenko), is dedicated to developing tissue models based on spheroids and organoids, including the in vitro glioblastoma model.
We utilize differentiated cells (endothelial, smooth muscle, fibroblasts, osteoblasts, and chondrocytes) or stem cells (derived from adipose tissue, bone marrow, Wharton’s jelly of the umbilical cord, or induced pluripotent stem cells) as the cellular component of our constructs. Cell growth, differentiation, and phenotypic maturation are sped up by mechanical stimulation in dynamic bioreactors. We also develop 3D bioprinting of biological and synthetic matrices that incorporate cells. Additionally, we introduce pre-vascularization into our tissue constructs, which is essential for the survival of the construct after implantation and for it to function properly as an in vitro tissue model.
Projects
Achievements
Top Czech Scientist of 2024
More information here: https://forbes.cz/lists/tcv-24/lucie-bacakova/
Isolation and culture of stem cells of the adipose tissue
Differentiated cells grow slower and have a limited viability in cell culture conditions. Isolation of differentiated cells from the patient does not eliminate the problem with other additive operations and damage of the other tissues of the patient. For example, for obtaining endothelial or the smooth muscle cells for reconstruction of blood vessels must remove part of subcutaneous veins from the patient. Stem cells from the patient’s body can be obtained still more simply, e.g. blood sampling (endothelial progenitor cells), puncture bone marrow of the bone tissue under the skin, e.g. from the hip bone, liposuction of fat tissue. Therefore, in collaboration with Hospital Motol we try to differentiate adipose stem cells into vascular or bone cells for the colonization of our constructs.
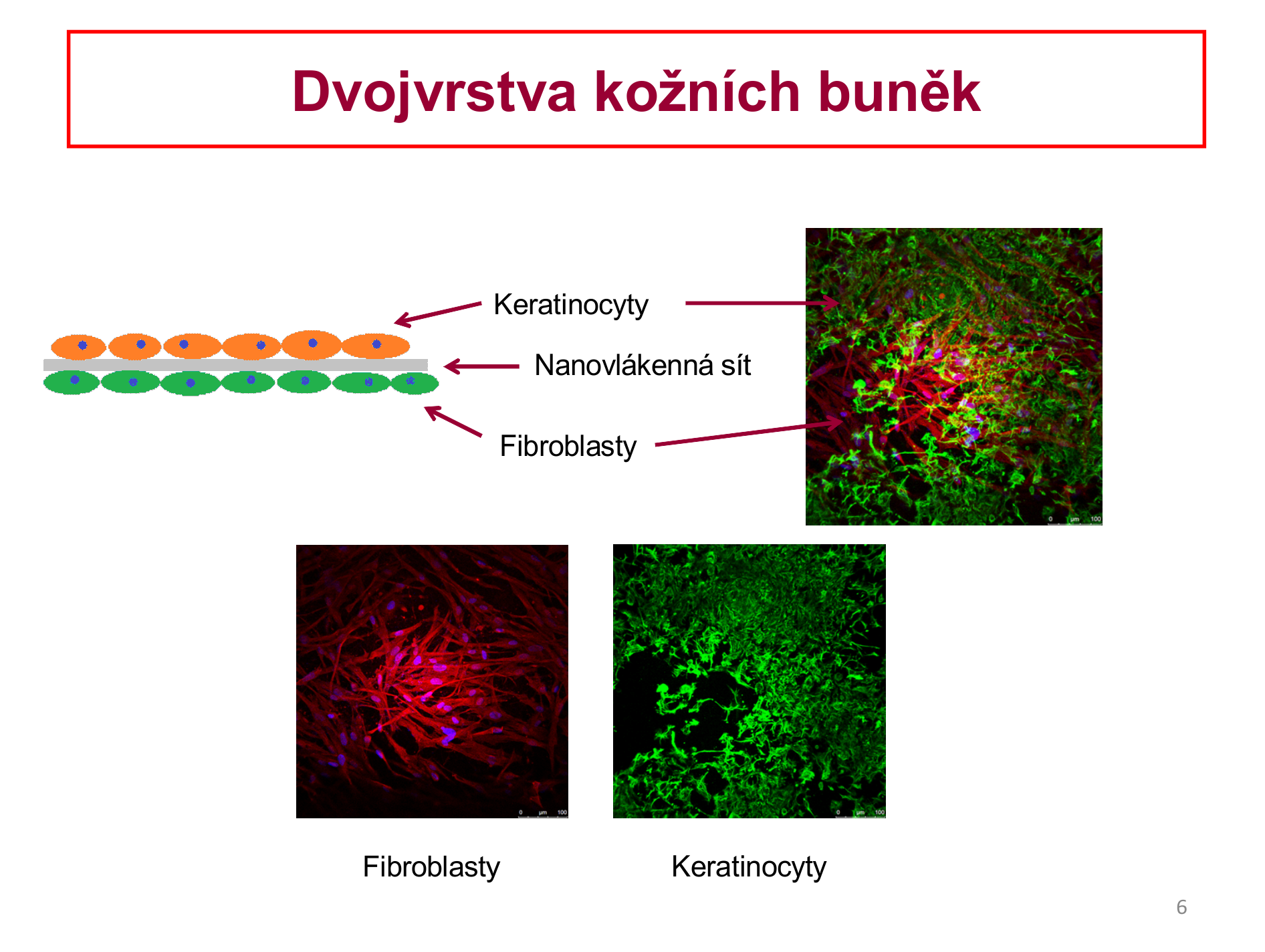
Skin tissue engineering
Construction of the bilayer structure of human keratinocytes (the main cell type of the epidermis) and fibroblasts (the main cell type of the dermis) to nanofiber membranes of polylactide.
Bačáková M, Musílková J, Riedel T, Stránská D, Brynda E, Žaloudková M, Bačáková L. The potential applications of fibrin-coated electrospun polylactide nanofibers in skin tissue engineering. International Journal of Nanomedicine. Roč. 11 (2016): 771-789, 2016, IF = 4.320
Bačáková M, Lopot F, Hadraba D, Varga M, Žaloudková M, Stránská D, Suchý T, Bačáková L. Effects of fiber density and plasma modification of nanofibrous membranes on the adhesion and growth of HaCaT keratinocytes. Journal of Biomaterials Applications. Vol. 29 (6): 837-853, 2015,IF = 1.988
Publications
Mekadim; Chahrazed - Mrázek; Jakub - Vodička; Martin - Ergang; Peter - Olša Fliegerová; Kateřina - Mahayri; Tiziana Maria - Chawengsaksophak; Kallayanee - Pácha; Jiří Impact of Sex; Gonadectomy; and Repeated Restraint Stress on Gut Microbiome in Mice. Molecular Neurobiology. 2025; 63(1); 80.
IF = 4.3
Sedlář; Antonín - Bojarová; Pavla - Kolář; František - Křen; Vladimír - Bačáková; Lucie Galectin-3 as a therapeutic target in pulmonary hypertension: Molecular mechanisms; drug development directions; and emerging clinical applications. Biomedicine & Pharmacotherapy. 2025; 193(Dec); 118756.
IF = 7.5
Havaldar; D. - Walter; J. - Starý; Zdeněk - Cvrček; L. - Gabor; R. - Mašek; K. - Filová; Elena - Staňková; Ľubica - Jeníková; Z. - Pawar; K. D. Mechanical and tribological behaviour of BaTiO3 nanoparticles reinforced UHMWPE nanocomposite for prospective load-bearing applications. Materials and Design. 2025; 256(August); 114349.
IF = 7.9
Hlukhaniuk; Anna - Swietek; Malgorzata Anna - Patsula; Vitalii - Janoušková; O. - Brož; Antonín - Malić; Marina - Kolodziej; A. - Weselucha-Birczynska; A. - Hodan; Jiří - Šlouf; Miroslav - Tokarz; W. - Zasońska; Beata Anna - Bystrianský; L. - Gryndler; M. - Bačáková; Lucie - Horák; Daniel Electrospun PCL mats modified with magnetic nanoparticles and tannic acid with antibacterial and possible antiosteosarcoma activity for bone tissue engineering and cancer treatment. ACS Biomaterials Science & Engineering. 2025; 11(7); 4315-4330.
IF = 5.5
Koper; F. - Grzywna; D. - Svobodová; Lucie - Sedlář; Antonín - Kobera; Libor - Riedel; Tomáš - Riedelová; Zuzana - Flis; A. - Beneš; Hynek - Pamula; E. - Bačáková; Lucie - Kasprzyk; W. P. Cross-linked poly(alkylene citrates) with L-glutathione for vascular tissue engineering: structure–property relationships and cell-type dependent response to oxidative stress. Journal of Materials Chemistry B. 2025; 13(34); 10552-10575.
IF = 5.7
GRANT PROJECTS
Newly awarded grant projects from 2026 onwards
Czech Science Foundation (GAČR), No. 26-21031S, „Novel MXene-based composite nanomaterials for tissue engineering“, duration 2026-2028. Principal Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci., Co-Investigator: Václav Švorčík, University of Chemistry and Technology, Prague
Czech Science Foundation (GAČR), No. 26-20451S, „Pulmonary Hypertension: A Multitarget Approach Using Tailored Galectin Inhibitors“, duration 2026-2028. Principal Investigator: Pavla Bojarová, Institute of Microbiology of the Czech Acad. Sci., Co-Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Czech Health Research Council (AZV), Ministry of Health of the Czech Republic, No. NW26-09-00043, „Innovative coatings for dental applications based on microdomains with encapsulated silver nanoparticles with permanent and/or light-induced bactericidal protection“, duration 2026-2029. Principal Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci., Co-Investigators: Jakub Siegel, University of Chemistry and Technology, Prague; Ivo Marek, Olomouc University Hospital; Josef Kučera, Charles University/First Faculty of Medicine.
Czech Health Research Council (AZV), Ministry of Health of the Czech Republic, No. NW26-08-00030, “Biocompatible Light-Responsive Materials for Catheters, Wound Dressings and Tissue Engineering“. Principal Investigator: Jan Marek, Faculty Hospital Hradec Králové, Co-Investigators: Elena Filová, Institute of Physiology of the Czech Acad. Sci., Jiří Mosinger, Charles University/Faculty of Science.
Present grant projects
Ministry of Education, Youth and Sports of the Czech Republic, INTER-EXCELLENCE, INTER-ACTION-LUAKR25, „Conductive Hydrogel Fibers for Cellular Stimulation and Tissue Engineering“, duration 2025-2028. Partners: Antonín Brož, Institute of Physiology of the Czech Acad. Sci.; Jinhwan Yoon, University of Seoul, Korea
Ministry of Education, Youth and Sports of the Czech Republic, co-funded by the European Union, OP JAC programme, project No. CZ.02.01.01/00/22_008/0004562 “Excellence in Regererative Medicine” (ExRegMed), duration 2023-2028. Principal Investigator: Pavla Jendelová, Institute of Experimental Medicine of the Czech Acad. Sci.; Co-Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.; leader of WP4 – Vascular Tissue Engineering; Elena Filova, IPHYS, leader of WP6 – Skin tissue engineering and wound healing
Czech Academy of Sciences, No. 2202, Praemium Academiae, “Regenerative medicine: cell interactions with biomaterials and tissue engineering”. Duration 2023-2028. Principal Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Czech Health Research Council (AZV), Ministry of Health of the Czech Republic, No. NU22-06-00016, „A novel tissue-engineered in vitro model for studying the pathogenesis and treatment of hypertrophic scars“, duration 2022-2025. Principal Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.; Co-Investigator: Tomáš Novotný, Krajská zdravotní, a.s., Masaryk Hospital, Ústí n. L.
Czech Health Research Council (AZV), Ministry of Health of the Czech Republic, No. NW25-10-00263, „Allogenic Multipotent Mesenchymal Stromal Cell Spheroids for Cartilage Regeneration: Preclinical Evaluation in Porcine Osteochondral Defect Model“, duration 2025-2028. Principal Investigator: Prof. MUDr. Vojtěch Havlas, PhD. Charles University/Second Faculty of Medicine, Co-Investigator: Dr. Yuriy Petrenko, Institute of Physiology of the Czech Acad. Sci.
Czech Academy of Sciences, Mobility Plus, No. ASRT-25-02 „Multifunctional Bioactive Composite Scaffolds for Drug and Ion Delivery and Hard and Soft Tissue Reconstruction“, duration 2025-2027. Partners: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.; Zainab Al-Rashidy, National Research Center, ASRT, Cairo, Egypt
Czech Academy of Sciences, Mobility Plus, No. UoM-24-02 „Versatile application of chitosan-based nanofibrous membranes for pre-vascularized tissue engineering“, duration 2024-2026. Partners: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.; Joel D. Bumgardner, University of Memphis, Memphis, Tennessee, USA.
Past grant projects 1997-2025
Funded by the EU
Project CarDia, National Institute for Research of Metabolic and Cardiovascular Diseases (Programme EXCELES, ID Project No. LX22NPO5104) – Funded by the European Union – Next Generation EU. Principal Investigator: Institute of Clinical and Experimental Medicine (IKEM), duration 2023-2025. Co-Investigator: Institute of Physiology of the Czech Acad. Sci. (Members of the laboratory of Biomaterials and Tissue engineering participated as professional colleagues; Lucie Bačáková: Activity 3.2.: Development of a tissue substitute for vascular wall reconstruction, Elena Filová: Activity 3.3.: Wound healing using “smart” nanofibrous covers releasing platelet lysate components and serving as carriers for delivery of autologous adipose tissue stem cells into wounds
Marie Curie Postdoctoral Fellowship for Andreu Blanquer, a member of our lab: „Piezoelectric Nanogenerators for skin wound healing“, ID 101003407, acronym ELECTROSKIN), duration 2020-2022.
Czech Science Foundation (GAČR):
Czech Science Foundation (GAČR), No. 22-00317S, „Galectin-3 as a target molecule for the reversal of vascular remodelling in hypoxic pulmonary hypertension“, duration 2022-2024. Principal Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci., Co-Investigator: Pavla Bojarová, Institute of Microbiology of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. 21-06065S, „New functionalized plasmon-based sensors as tools for cell monitoring and advanced tissue engineering“, duration 2021-2023, Principal Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci., Co-Investigator: Václav Švorčík, University of Chemistry and Technology in Prague
Czech Science Foundation (GAČR), No. 20-01641S, “Degradable nanocellulose as a novel temporary scaffold for tissue engineering”, duration 2020-2022, Principal Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci., Co-Investigator: Václav Švorčík, University of Chemistry and Technology in Prague
Czech Science Foundation (GAČR), No. 20-01570S, “Improved osseointegration of bone implants with the use of ferroelectric coatings”, duration 2020-2022, Principal Investigator: Marta Vandrovcová, Institute of Physiology of the Czech Acad. Sci.; Co-Investigators: Přemysl Vaněk, Institute of Physics of the Czech Acad. Sci., Ladislav Cvrček, Faculty of Mechanical Engineering, Czech Technical University, Prague.
Czech Science Foundation (GAČR), No. 20-07015S, “Magnetic Composite Materials for Non-Invasive Cell Stimulation in Tissue Engineering”, duration 2020-2022, Principal Investigator: Antonín Brož, Institute of Physiology of the Czech Acad. Sci., Co-Investigator: Daniel Horák, Institute of Macromolecular Chemistry of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. 19-02891S, “Rational development of smart prosthetic material: Silicalite-1 coating on TiAlV alloy“, duration 2019-2021. Principal Investigator: Ivan Jirka, Heyrovský Institute of Physical Chemistry of the Czech Acad. Sci.; Co-Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. 18-12774S, “Plasma polymers deposited on nanofibrous membranes for vascular tissue engineering”, duration 2018-2020. Principal Investigator: Lenka Zajíčková, Masaryk University, Central European Institute of Technology (CEITEC), Brno, Czech Republic, Co-Investigator: Ivana Kopová-Němčáková, Institute of Physiology of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. 18-01163S, “Self-endothelialization of novel bioactive surfaces of decellularized vascular grafts“, duration 2018-2020, Principal Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci., Co-Investigators: Vladimír Křen, Institute of Microbiology of the Czech Acad. Sci., Tomáš Riedel, Institute of Macromolecular Chemistry of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. 17-02448S, „Improved growth of human skin cells on biomimetic nanofibrous matrices for active wound healing“, duration 2017-2019. Principal Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci, Co-Investigators: David Lukáš, Technical University in Liberec, Vladimír Král, 1st Faculty of Medicine, Charles University.
Czech Science Foundation (GAČR), No. 17-00885S, „Nanostructured cellulose-based scaffolds with tunable properties for tissue engineering“, duration 2017-2019. Principal Investigator: Václav Švorčík, University of Chemistry and Technology in Prague; Co-Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. 16-02681S, „Silicalite Nanofilms with Tunable Properties as a Perspective Biomaterial“, duration 2016-2018. Principal Investigator: Ivan Jirka, Heyrovský Institute of Physical Chemistry of the Czech Acad. Sci., Co-Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. 15-01558S5 „Electroactive films on titanium alloy substrates for surface modification of bone implants“, duration 2015-2017. Principal Investigator: Petr Špatenka Czech Technical University, Co-Investigator: Marta Vandrovcová, Institute of Physiology of the Czech Acad. Sci.
Czech Science Foundation (GAČR) No. 15-05864S, „Biocompatibility of doped DLC layers prepared by hybrid laser technology“, duration 2015-2017. Principal Investigator: Miroslav Jelínek, Institute of Physics of the Czech Acad. Sci., Co-Investigator: Elena Filová, Institute of Physiology of the Czech Acad. Sci.
Czech Science Foundation (GAČR) No. 14-04790S (108/14/04790) „Engineering Bulk and Surface of Diamond Nano-Objects for Biomedicine”, duration 2014-2016. Principal Investigator: Štěpán Potocký, Institute of Physics of the Czech Acad. Sci., Co-Investigator: Lubica Staňková, Institute of Physiology of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. P108/12/G108, Center of Excellence: „Preparation, modification and characterization of materials by radiation“, duration 2012-2018. Principal Investigator: Václav Švorčík, University of Chemistry and Technology, Prague; Co-Investigator Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. P107/12/1025 „Complex investigation of advanced beta-titanium alloys designed for biomedicine“, duration 2012-2015. Principal Investigator: Miloš Janeček, Faculty of Mathematics and Physics, Charles University, Prague; Co-Investigator: Marta Vandrovcová, Institute of Physiology of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. P108/12/1168 “Carbon nanolayers, nanostructures and nanoparticles on substrates for potential application in medicine and electronics”, duration 2012-2016. Principal Investigator: Václav Švorčík, University of Chemistry and Technology in Prague; Co-Investigator Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. P108/11/1857 “Polymeric biomaterials for vascular tissue engineering“, duration 2011-2014. Principal Investigator: František Rypáček, Institute of Macromolecular Chemistry of the Czech Acad. Sci., Co-Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. P108/11/0794 “Visualization of collagen production in osteogenic cells cultivated on nanocrystalline diamond films“, duration 2011-2013. Principal Investigator: Lucie Kubínová, Institute of Physiology of the Czech Acad. Sci.; Co-Investigator: Alexander Kromka, Institute of Physics of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. P107/11/1856 “Metal-fullerene nanocomposites and their biological applications“, duration 2011-2013. Principal Investigator: Jiří Vacík, Nuclear Physics Institute of the Czech Acad. Sci., Řež near Prague; Co-Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. P108/10/1106 “The structure and properties of modified polymers for tissue engineering“, duration 2010-2014. Principal Investigator: Václav Švorčík, University of Chemistry and Technology in Prague, Co-Investigators: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.; Zdeňka Kolská, Purkinje Univ., Ústí nad Labem
Czech Science Foundation (GAČR), No. P108/10/1858 “Stability and Biocompatibility of Surface of Oxidic Layer on a Monophasic TiNb Alloy“, duration 2010-2012. Principal Investigator: Vladimír Starý, Czech Technical University in Prague; Co-Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. 106/09/1000 “Bioinspired Nanocomposite Structures for Bone Tissue Regeneration”, duration 2009-2012. Principal Investigator: Karel Balík, Institute of Rock Structure and Mechanics of the Czech Acad. Sci., Co-Investigators: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci., Marcela Munzarová, Elmarco s.r.o., Liberec, Dana Kubies, Institute of Macromolecular Chemistry of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. 305/08/0108 “Recovery from chronic hypoxia of lung and effects of hypoxia in placenta. Pathophysiological basis for therapy“, duration 2008-2012. Principal Investigator: Jan Herget, 2nd Faculty of Medicine, Charles University; Co-Investigators: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. 204/06/0225 „Adhesion, Growth and Differentiation of Bone and Vascular Cells on Carbon Allotropes“, duration 2006-2008. Principal Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci., Co-Investigators: Jiří Vacík, Nuclear Physics Institute of the Czech Acad. Sci., Řež near Prague; Václav Švorčík, University of Chemistry and Technology in Prague
Czech Science Foundation (GAČR), No. 101/06/0226 „Surface-modified carbon composites as potential implants into human body“, duration 2006-2008. Principal Investigator: Rudolf Dvořák, Faculty of Mechanical Engineering, Czech Technical University in Prague, Co-Investigators: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. 106/06/1576 „Porous Composite Materials with Polyamide Reinforcement and Siloxane matrix with Nano-Hydroxyapatite as Biomaterials“, duration 2006-2008. Principal Investigator: Karel Balík, Institute of Rock Structure and Mechanics of the Czech Acad. Sci., Co-Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. 305/05/0672 „Dynamics and mechanisms of the chronic hypoxia-induced alterations in pulmonary and placental circulations“, duration 2005-2007. Principal Investigator: Václav Hampl, 2nd Faculty of Medicine, Charles University; Co-Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. 106/99/0626”, Influence of Surface Properties of Composite Materials on Their Biocompatibility”, duration 1999-2001. Principal Investigator: Vladimír Starý, Faculty of Mechanical Engineering, Czech Technical University in Prague; Co-Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Czech Science Foundation (GAČR), No. 305/97/S070, ”The Mechanism of Hypoxic Pulmonary Hypertension in Newborns and Adults”, duration 1997-2002. Principal Investigator: Jan Herget, 2nd Faculty of Medicine, Charles University; Co-Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Czech Health Research Council (AZV), Ministry of Health of the Czech Republic:
Czech Health Research Council (AZV), Ministry of Health of the Czech Republic, No. NU20-08-00208 “Novel vascularized stem-cell based constructs for soft and hard tissue engineering”, duration 2020-2023, Principal Investigator: Jana Musílková, Institute of Physiology of the Czech Acad. Sci., Co-Investigators: Martin Molitor, Nemocnice Na Bulovce Hospital, Prague; Miloš Beran, Food Research Institute in Prague, Petr Slepička, University of Chemistry and Technology in Prague; Zdeňka Kolská, J.E. Purkinje University, Ústí nad Labem.
Czech Health Research Council (AZV), Ministry of Health of the Czech Republic, No. NV19-06-00355, “Safety and efficacy evaluation of allogeneic mesenchymal stem cells derived from umbilical cord tissue in repair of cartilage defects: preclinical study on animal model“, duration 2019-2022, Principal Investigator: Vojtěch Havlas, 2nd Faculty of Medicine, Charles University; Co-Investigator: Irena Vacková, Institute of Physiology of the Czech Acad. Sci.
Czech Health Research Council (AZV), Ministry of Health of the Czech Republic, No. NV19-02-00068, „Bioartificial cardiovascular patches and vessels from porcine collagen reinforced with nano/microfibers using stem cells and dynamic culture“, duration 2019-2022. Principal Investigator: Jan Pirk, Institute of Clinical and Experimental Medicine (IKEM); Co-Investigators: Elena Filová, Institute of Physiology of the Czech Acad. Sci., Monika Šupová, Institute of Rock Structure and Mechanics of the Czech Acad. Sci., Peter Kneppo, Faculty of Biomedical Engineering, Czech Technical University
Czech Health Research Council (AZV), Ministry of Health of the Czech Republic, No. NV18-02-00422, “New materials for cardiovascular surgery based on modified decellularized tissues“, duration 2018-2021, Principal Investigator: Jan Pirk, Institute of Clinical and Experimental Medicine (IKEM), Prague; Co-Investigators: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci., Peter Kneppo, Faculty of Biomedical Engineering, Czech Technical Univ., Serhiy Forostyak, PrimeCell Bioscience, a.s.
Czech Health Research Council (AZV), Ministry of Health of the Czech Republic, No. NV18-01-00332, “Treatment of diabetic wounds using nanofibrous dressings releasing platelet lysate components“, duration 2018-2021, Principal Investigator: Elena Filová, Institute of Physiology of the Czech Acad. Sci., Pavel Klein, Faculty of Medicine in Pilsen, Charles University, Věra Jenčová, Technical University Liberec, Eduard Brynda, Institute of Macromolecular Chemistry.
Czech Health Research Council (AZV), Ministry of Health of the Czech Republic, No. 15-32497A „Bioactive nanostructured surfaces for histocompatible implants“, duration 2015-2018. Principal Investigator: David Sedmera, Institute of Physiology of the Czech Acad. Sci., Co-Investigators: Bohuslav Rezek, Institute of Physics of the Czech Acad. Sci.; Petr Slepička, University of Chemistry and Technology in Prague, Václav Mandys, 3rd Faculty of Medicine, Charles University
Czech Health Research Council (AZV), Ministry of Health of the Czech Republic, No. 15-33018A, „Application of adipose tissue-derived stem cells obtained by liposuction in tissue engineering“, duration 2015-2018 Principal Investigator: Martin Molitor, Na Bulovce Hospital, Prague, Co-Investigators: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.; Václav Švorčík, University of Chemistry and Technology in Prague, Alexander Kromka, Institute of Physics of the Czech Acad. Sci.
Czech Health Research Council (AZV), Ministry of Health of the Czech Republic, No. 15-29153A, „Development of an aortic heart valve based on pericardium using primary and stem cells and mechanical loading in a bioreactor“, duration 2015-2018. Principal Investigator: Elena Filová, Institute of Physiology of the Czech Acad. Sci.; Co-Investigator: Jan Pirk, Institute of Clinical and Experimental Medicine (IKEM), Prague
Internal Grant Agency, Ministry of Health of the Czech Republic, No. NT13297-4/2012, duration 2012-2015. “Augmentation of biological fixation of titanium implants by biomimetic hydrogel coatings enriched with bioactive compounds: The effect on adhesion and synthetic activity of osteoblasts and cells of immune response. Study in vitro.” Principal Investigator: Lucie Himmlová, General Teaching Hospital in Prague, Co-Investigators: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.; Dana Kubies, Institute of Macromolecular Chemistry of the Czech Acad. Sci.
Internal Grant Agency, Ministry of Health of the Czech Republic, No. NT 11 270 01, “Tissue engineering of autologous pericardial valve replacements”, duration 2012-2015. Principal Investigator: Jan Pirk, IKEM, Prague; Co-Investigator: Elena Filová, Institute of Physiology of the Czech Acad. Sci.
Internal Grant Agency, Ministry of Health of the Czech Republic, No. NR 9358-3 „Local medicamentous inhibition of the neointimal proliferation in venous grafts after their interposition into the abdominal aorta in rats“, duration 2007-2009. Principal Investigator: MUDr. Ivo Skalský, IKEM, Prague; Co-Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Technology Agency of the Czech Republic
Technology Agency of the Czech Republic (TAČR), Programme DELTA 2, No. TM01000046, “Modular 3D bioprinting system for printing biocompatible hydrogel and polymer scaffolds for tissue engineering”, duration 2020-2022, Principal Investigator: Serhiy Forostyak, PrimeCell Bioscience, a.s., Co-Investigator: Roman Matějka, Institute of Physiology of the Czech Acad. Sci.; Taiwanese partner: Amber Shen, Life Star International Limited.
Technology Agency of the Czech Republic (TAČR), No. TA04011345 „Small-diameter vascular prostheses seeded with endothelial cells and bone marrow-derived stem cells in a bioreactor“, duration 2014-2017, Principal Investigator: Eva Matějková, National Center for Tissues and Cells a.s. Co-Investigator: Elena Filová, Institute of Physiology of the Czech Acad. Sci., Jozef Rosina, Czech Technical University in Prague (FBME Kladno)
Technology Agency of the Czech Republic (TAČR), No. TA04010065 „Matrix systems for healing of skin defects for human and veterinary use“, duration 2014-2017. Principal Investigator: Tomáš Sopuch, Holzbecher, spol. s r. o.; Co-Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Technology Agency of the Czech Republic (TAČR), No. TA04011214 “Application of electrochemical anodization to achieve surface modification of titanium alloys”, duration 2014-2017. Principal Investigator: Roman Gabor, VÚHŽ a.s., Dobrá, Czech Republic; Co-Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Technology Agency of the Czech Republic (TAČR), No. TA01011141 “Comprehensive research into endoprostheses with improved functional properties based on beta titanium alloys”, duration 2011-2014. Principal Investigator: Jaroslav Fencl, Beznoska s.r.o., Kladno; Co-Investigators: Miloš Janeček, Faculty of Mathematics and Physics, Charles University, Prague; Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Technology Agency of the Czech Republic (TAČR), No. FT-TA2/013, “Resorbable gel assemblies for affecting postoperative adhesions, spinal surgery and compressions with healing effects“, duration 2005-2007. Principal Investigator: Michal Bartoš, VÚOS a.s., Pardubice, one of the co-investigators: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Ministry of Education, Youth and Sports (MEYS)
MEYS, Programme „Healthy and High-Quality Life“, No. 2B06173, “High value-added materials and products from agricultural and food waste“, duration 2006-2011. Principal Investigator: Miloš Beran, Food Research Institute, Prague; one of the co-investigators: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
OPVK, No. CZ.1.07/2.3.00/30.0025 „Centre of Biomedical Research“; Lucie Bačáková (mentor), Jana Lišková (postdoc)
Ministry of Industry and Trade of the Czech Republic (MPO)
MPO-TIP, No. FR-TI3/088 “Development of implants, instruments, and fixators with antibacterial coatings based on nanostructured surfaces”, duration 2011-2013, Principal Investigator: Zdeněk Čejka, Prospon s.r.o., Kladno, Co-Investigator: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
MPO Programme „Sustainable prosperity“, No 2A-1TP1/073, „New pharmaceutical products based on polysaccharides“, duration 2006-2011. Principal Investigator: Michal Bartoš, VÚOS a.s., Pardubice, one of the co-investigators: Lucie Bačáková, Institute of Physiology, Acad. Sci. CR.
Czech Academy of Sciences
Grant Agency of the Academy of Sciences of the Czech Republic (GA AV ČR), No. IAAX00100902 „Electronically and chemically optimized nanocrystalline diamond structures for bio-applications“, duration 2009-2012. Principal Investigator: Milan Vaněček, Institute of Physics of the Acad. Sci. CR; Co-Investigator: Lucie Bačáková, Institute of Physiology, Acad. Sci. CR.
Grant Agency of the Academy of Sciences of the Czech Republic (GA AV ČR), No. IAA400320901 „Gold and silver surfaces modified with functional derivatives of boron hydrides“, duration 2009-2011. Principal Investigator: Tomáš Baše, Institute of Inorganic Chemistry of the Acad. Sci. CR; Co-Investigator: Lucie Bačáková, Institute of Physiology, Acad. Sci. CR.
Grant Agency of the Academy of Sciences of the Czech Republic (GA AV ČR), Programme „Nanotechnology for Society“, No. KAN101120701 „Nanocomposite layers and nanoparticles created in low-pressure plasma for surface modifications“, duration 2007-2011. Principal Investigator: Hynek Biederman, Faculty of Mathematics and Physics, Charles University, Prague; one of the co-investigators: Lucie Bačáková, Institute of Physiology of the Acad. Sci. CR
Grant Agency of the Academy of Sciences of the Czech Republic (GA AV ČR), Programme „Nanotechnology for Society“, No. KAN400480701 „Carbon- and polymer-based nanostructures for applications in bioelectronics and medicine“, duration 2007-2011. Principal Investigator: Jiří Vacík, Nuclear Physics Institute of the Czech Acad. Sci., Řež near Prague; Co-Investigators: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.; Václav Švorčík, University of Chemistry and Technology in Prague; Tomáš Baše, Institute of Inorganic Chemistry of the Acad. Sci. CR.
Grant Agency of the Academy of Sciences of the Czech Republic (GA AV ČR), Programme “Support for targeted research projects”, No. 1QS500110564, “New hybrid bioartificial prostheses constructed by methods of tissue engineering.“, duration 2005-2009. Principal Investigators: Lucie Bačáková, Institute of Physiology of the Acad. Sci. CR.; Co-Investigator: František Rypáček, Institute of Macromolecular Chemistry of the Acad. Sci. CR.
Grant Agency of the Academy of Sciences of the Czech Republic (GA AV ČR) No. A 5011301 „Vascular cells on synthetic polymers with micro-patterned surfaces“, duration 2003-2007. Principal Investigators: Lucie Bačáková, Institute of Physiology of the Acad. Sci. CR.; Co-Investigators: Václav Švorčík, University of Chemistry and Technology in Prague; Vladimír Hnatowicz, Nuclear Physics Institute of the Acad. Sci. CR.
Grant Agency of the Academy of Sciences of the Czech Republic (GA AV ČR) No. A7011908 ”Increasing the adhesive capacity of cells by modifying the surfaces of polymer carriers”, duration 1999-2001. Principal Investigators: Lucie Bačáková, Institute of Physiology of the Acad. Sci. CR.; Co-Investigators: Václav Švorčík, University of Chemistry and Technology in Prague; Vladimír Hnatowicz, Nuclear Physics Institute of the Acad. Sci. CR.
Grant Agency of Charles University (GA UK)
Grants for PhD students:
GA UK No. 295623, Marina Malić: 3D in vitro model endochondrální osifikace pro testování biomateriálů (3D in vitro model of endochondral ossification for testing biomaterials)
GA UK No. 410121, Martina Doubková: Produkce mezibuněčné hmoty buňkami izolovanými z pes equinovarus a testování látek ovlivňujících zesíťování kolagenu v makromolekulárně nahloučeném prostředí (Production of extracellular matrix by cells isolated from Pes equinovarus and testing of substances affecting collagen cross-linking in a macromolecularly crowded environment)
GA UK No. 756218, Júlia Pajorová (Tomšů): Studium interakcí kožních a kmenových buněk s modifikovanými nanovlákennými materiály pro konstrukci kompletní kožní náhrady (Study of interactions between skin and stem cells with modified nanofiber materials for the construction of complete skin replacements)
GA UK No. 642217, Martina Trávníčková: Studium diferenciace kmenových buněk tukové tkáně směrem k cévním hladkým svalovým buňkám pro účely cévního tkáňového inženýrství (Study of the differentiation of adipose tissue stem cells into vascular smooth muscle cells for the purposes of vascular tissue engineering)
GA UK No. 38214, Markéta Bačáková (Zikmundová): Kolonizace modifikovaných nanovlákenných membrán kožními buňkami (Colonization of modified nanofibrous membranes with skin cells).
Standard project:
GA UK No. 188/98/C ”The effect of non-enzymatic modifications of collagen on the adhesion and growth of vascular smooth muscle cells in culture”, duration 1998-2000. Principal Investigator: Jan Herget, 2nd Faculty of Medicine, Charles University; Co-Investigators: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Other (international) grant projects
Mobility Plus, No. UofM-20-01, “Novel chitosan-based angiogenic nanofibrous scaffolds for skin tissue engineering”, duration 2020-2022. Czech partner: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci., American Partner: Prof. Joel D. Bumgardner, University of Memphis, Memphis, Tennessee, USA
Mobility for postdoctoral researcher Marta Vandrovcová (IPHYS), University of Malaga, Spain, 2018-2020. Topic: Surface modifications of hydroxyapatite with biomimetic peptides for improved osteogenesis and osseointegration.
International Mobility of Researchers, ID MSM200111802, „Microenvironmental regulation of vascular network formation and patterning“, Jana Zárubová (IPHYS), postdoctoral researcher, University of California, Los Angeles (UCLA), USA, 2017
BARRANDE No. 2005-06-036-1 „Vascular endothelial and smooth muscle cells on synthetic polymers with bioactive ligands for tissue engineering“, duration 2005-2006. Partners: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.; Prof. Laurence Bordenave, Université Victor Segalen, Bordeaux, France
Project COST 527, Action 130, ID: OC/PR/00680, in 2005 1P05OC012; „In vitro biocompatibility and antimicrobial properties of biofunctional substrates coated with hard hydrocarbon polymer composite layers“, duration 2002-2005: Principal Investigator for the Action: Lucie Bačáková, Institute of Physiology of the Czech Acad. Sci.
Book Chapters, Application Results
Book chapters
Bačáková, L., Zikmundová, M., Pajorová, J., Brož, A., Filová, E., Blanquer, A., Matějka, R., Štěpanovská, J., Mikeš, P., Jenčová, V., Kuželová Košťáková, E., Sinica, A. Nanofibrous Scaffolds for Skin Tissue Engineering and Wound Healing Based on Synthetic Polymers. In: STOYTCHEVA, Margarita, ZLATEV, Roumen, eds. Applications of Nanobiotechnology. London: IntechOpen, 2020, s. 1-29. ISBN 978-1-78985-978-2. DOI: 10.5772/intechopen.88744.
Bačáková, L., Pajorová, J., Zikmundová, M., Filová, E., Mikeš, P., Jenčová, V., Kuželová Košťáková, E., Sinica, A. Nanofibrous Scaffolds for Skin Tissue Engineering and Wound Healing Based on Nature-Derived Polymers. In: KHALIL, Islam, ed. Current and Future Aspects of Nanomedicine. London: IntechOpen, 2020, s. 1-30. ISBN 978-1-78985-870-9. DOI: 10.5772/intechopen.88602.
Bacakova L, Travnickova M, Filova E, Matejka R, Stepanovska J, Musilkova J, Zarubova J, Molitor M. The Role of Vascular Smooth Muscle Cells in the Physiology and Pathophysiology of Blood Vessels. In: Muscle Cell and Tissue: Current Status of Research Field, Ed. Kunihiro Sakuma. IntechOpen, London, United Kingdom, chapter 12, pages 229-256; 2018. ISBN 978-953-51-6092-2; http://dx.doi.org/10.5772/intechopen.77115
Bacakova L, Travnickova M, Filova E, Matejka R, Stepanovska J, Musilkova J, Zarubova J, Molitor M. Vascular Smooth Muscle Cells (VSMCs) in Blood Vessel Tissue Engineering: The Use of Differentiated Cells or Stem Cells as VSMC Precursors. In: Muscle Cell and Tissue: Current Status of Research Field, Ed. Kunihiro Sakuma. IntechOpen, London, United Kingdom, chapter 14, pages 289-308; 2018. ISBN 978-953-51-6092-2; http://dx.doi.org/10.5772/intechopen.77108
Bacakova L, Bacakova M, Pajorova J, Kudlackova R, Stankova L, Musilkova J, Potocky S, Kromka A. Nanofibrous Scaffolds as Promising Cell Carriers for Tissue Engineering. Chapter 3 in the book Nanofiber Research – Reaching New Heights. Edited by Mohammed Muzibur Rahman and Abdullah M. Asiri, ISBN 978-953-51-2529-7, Print ISBN 978-953-51-2528-0, pp. 29-54, 2016. Publisher: IntechOpen, London, United Kingdom, doi: 10.5772/63707
Bacakova L, Filova E, Liskova J, Kopova I, Vandrovcova M, Havlikova J. Nanostructured materials as substrates for the adhesion, growth, and osteogenic differentiation of bone cells. In: Nanobiomaterials in Hard Tissue Engineering. Applications of Nanobiomaterials. Volume 4, Ed. A. M. Grumezescu, Elsevier Inc., William Andrew Publishing, Oxford, Cambridge; Chapter 4, pp. 103-153, ISBN 978-0-323-42862-0; 2016
Bacakova L, Broz A, Liskova J, Stankova L, Potocky S, Kromka A. The Application of Nanodiamond in Biotechnology and Tissue Engineering. Chapter 3 in the book Diamond and Carbon Composites and Nanocomposites. Edited by Mahmood Aliofkhazraei, ISBN 978-953-51-2454-2, Print ISBN 978-953-51-2453-5, pp. 59-88, 2016. Publisher: IntechOpen, London, United Kingdom, doi: 10.5772/61410
Bačáková L, Novotná K, Sopuch T, Havelka P. Cell Interaction with Cellulose-Based Scaffolds for Tissue Engineering – A Review. In: Cellulose and Cellulose Derivatives: Synthesis, Modification, Nanostructure and Applications. Edited by I.H. Mondal, Nova Science Publishers, Inc. Hauppauge, New York, USA, 2015, pp 341-375. ISBN 978-1-63483-571-8.
Bačáková L, Kopová I, Vacík J, Lavrentiev V. Interaction of fullerenes and metal-fullerene composites with cells. In: Fullerenes: Chemistry, Natural Sources and Technological Applications. Edited by S.B. Ellis, Nova Science Publishers, Inc., Hauppauge, New York, USA, 2014, pp 1-33. ISBN 978-1-63321-386-9.
Bačáková L, Grausová L, Vacík J, Kromka A, Biederman H, Choukourov A, Starý V: Nanocomposite and nanostructured carbon-based films as growth substrates for bone cells. In: Advances in Diverse Industrial Applications of Nanocomposites. (Ed. Boreddy Reddy), Intech, Open Access Publisher, str. 399-435, 2011; ISBN 978-953-307-202-9
Balík K, Suchý T, Sucharda Z, Černý M, Šupová M, Rýglová Š, Bačáková L, Filová E, Sedláček R: Výzkum kompozitních materiálů jako náhrad kostních štěpů pro aplikace ve formě jader meziobratlových rozpěrek. In: Interdisciplinární výzkum řešení problematiky páteřních náhrad, Edice monografií České společnosti pro biomechanik, ČVUT FS, Ústav mechaniky, Laboratoř biomechaniky člověka, str. 106-115, 2010; ISBN 978-80-01-04494-0
Bačáková L, Grausová L, Vandrovcová M, Vacík J, Frazcek A, Blazewicz S, Kromka, A, Vaněček M, Nesládek M, Švorčík V, Vorlíček V, Kopeček M: Carbon nanoparticles as substrates for cell adhesion and growth. In: Encyclopedia of Nanotechnology. (Ed. Carlson ED), Nova Science Publishers, Inc., 400 Oser Ave, Ste 1600, Hauppauge, NY 11788-3635 USA; Vols. 1 and 2, str. 1027-1094, 2009; ISBN: 978-1-60692-079-4
Bačáková L, Švorčík V: Cell colonization control by physical and chemical modification of materials. In: Cell Growth Processes: New Research (Ed. Daiki Kimura), Nova Science Publishers, Inc., Hauppauge, New York, USA; 2008, str. 5-56, ISBN 978-1-60456-123-6
Bačáková L, Grausová L, Vandrovcová M, Vacík J, Frazcek A, Blazewicz S, Kromka, A, Vaněček M, Nesládek M, Švorčík V, Kopeček M: Carbon nanoparticles as substrates for cell adhesion and growth. In: Nanoparticles: New Research (Ed. Simone Luca Lombardi), Nova Science Publishers, Inc., Hauppauge, New York, USA; 2008, str. 39-107, ISBN 978-1-60456-704-5
Patents
Czech patents:
Fencl J, Janecek M, Strasky J, Harcuba P, Havlikova J, Bacakova L: Joint implant and mode of its fabrication. Czech patent, Industrial Property Office of the CR, accepted in 2014, ID 304445.
Brynda E, Houska M, Syková E, Jendelová P, Dyr JE, Filová E, Riedel T, Chlupáč J, Lesný P, Bačáková L: Způsob přípravy regulovaných vrstev fibrinu na pevných površích. (Mode of preparation of regulated fibrin layers on solid surfaces) Institute of Macromolecular Chemistry AS CR, v.v.i. and Institute of Experimental Medicine of the ASCR, v.v.i. and Institute of Physiology AS CR, v.v.i. and The Institute of Hematology and Blood Transfusion. Praha: Industrial Property Office, 2008. No: 299687. Accepted: 12.09.2008.
EU Patent:
Rezek B, Michalíková L, Kromka A, Kalbáčová M, Kmoch S, Grausová L, Bačáková L, Vaněček M, Kočka J: Method of making arranged cell structures. Application No. 09761298, Z 7584, PV2008-355, EP 2288699. Accepted and validated: 2011.
Utility models
Elena Filová, Yu-Chieh Wu, Lucie Bačáková, Šárka Hauzerová, Věra Jenčová, Maxim Lisnenko, David Lukáš, Jana Mullerová, Kateřina Strnadová, Silvie Rimpelová, Nikola Slepičková, Petr Slepička. Polymerní nanovlákenná membrána. (Polymeric nanofibrous membrane.) Utility model No. 38802, date of acceptance 16.09.2025, application number: 2025-43054, date of application 06.02.2025.
Elena Filová, Yu-Chieh Wu, Lucie Bačáková, Šárka Hauzerová, Věra Jenčová, Maxim Lisnenko, David Lukáš, Jana Mullerová, Kateřina Strnadová, Silvie Rimpelová, Nikola Slepičková, Petr Slepička. Polymerní nanovlákenná membrána. (Polymeric nanofibrous membrane.) Utility model No. 38796, date of acceptance 16.09.2025, application number: 2025-42594, date of application 06.02.2025.
Dominik Fajstavr, Petr Slepička, Nikola Slepičková Kasálková, Jana Musílková: Plazmaticky a/nebo laserově modifikovaná polymerní folie jako substrát pro růst kmenových ASC buněk. (Plasma- and/or laser-modified polymer film as a substrate for the growth of stem ASC cells.) Utility model No. CZ 37393 U1, Industrial Property Office, Prague, CZ, accepted 25.10.2023. Owners: University of Chemistry and Technology in Prague; Institute of Physiology CAS
Miloš Beran, Jana Musílková, Antonín Sedlář: Polymerní konstrukty s biometrickými charakteristikami pro inženýrství kostních tkání (Polymeric constructs with biometric characteristics for bone tissue engineering). Utility model No. 37056, Industrial Property Office, Prague, CZ, accepted 23.05.2023. Owners: Food Research Institute, Prague; Institute of Physiology CAS
Miloš Beran, Jana Musílková, Antonín Sedlář: Pěny s hierarchickou porézní strukturou. (Foams with hierarchical pore structure.) Utility model No. 36870, Industrial Property Office, Prague, CZ, accepted 24.02.2023. Owners: Food Research Institute, Prague; Institute of Physiology CAS
Bačáková, Lucie – Filová, Elena – Pražák, Šimon – Vacková, Irena – Suchý, Tomáš – Šupová, Monika – Šepitka, J. – Procházková, R. – Jenčová, V. – Kuželová Košťáková, E. – Lisnenko, M. – Lukáš, D. – Valtera, J. Vyztužený kompozitní hydrogel s buňkami. [Reinforced composite hydrogel with cells.] 2023. Utility model No. 37438, approved: 01.11.2023. Owners: Institute of Physiology, Czech Academy of Sciences – Technical University of Liberec – Institute of Rock Structure and Mechanics, Czech Academy of Sciences – Czech Technical University in Prague – Regional Hospital Liberec, a.s.
Jan Falk Lipenský, Michal Zahradníček, Karel Hanzálek, Roman Matějka, Jana Štěpanovská: Modulární 3D tiskárna pro přímý biotisk hydrogelů s buněčnou suspenzí. (Modular 3D printer for direct bioprinting of hydrogels with cell suspension.) Utility model No. 37203, Industrial Property Office, Prague, CZ, approved, 25.07.2023. Owners: PrimeCell Bioscience, a.s., Ostrava, Poruba; Institute of Physiology, Czech Academy of Sciences, Praha 4, Krč
Filová, Elena – Musílková, Jana – Eckhardt, Adam – Hadraba, Daniel – Vondrášek, David – Pražák, Šimon – Bačáková, Lucie – Chvátil, David – Olšanský, Václav – Koňařík, M. – Pirk, J. Recelularizovaný perikard pro kardiovaskulární náhrady. [Recellularized pericardium for cardiovascular replacements.] 2022, Approved: 28.06.2022. ID: 36181. Owners: Institute of Physiology, Czech Academy of Sciences – Nuclear Physics Institute, Czech Academy of Sciences – Institute of Clinical and Experimental Medicine, Czech Academy of Sciences.
Karel Hanzálek, Roman Matějka, Jana Štěpanovská, S. Forostyak, J. F. Lipenský: Multikanálový směšovač pro biotisk hydrogelů s buněčnou suspenzí. (Multichannel mixer for bioprinting hydrogels with cell suspension.) Utility model No. 35784, Industrial Property Office, Prague, CZ, accepted 08.02.2022. Owners: Institute of Physiology, Czech Academy of Sciences – PrimeCell Bioscience, a. s.
Matějka R, Štěpanovská J, Kneppo P, Pražák Š, Bačáková L, Koňařík M, Chlupáč J, Pirk J. Kultivační komora pro tlakovou stimulaci v kultivačních jamkách. (Culture chamber for pressure stimulation in culture wells). Utility model, Industrial Property Office, Prague, Czech Republic;, submitted on 20. 01. 2020, registered under No. PUV 2020-37152, approved on 14. 04. 2020, No. 33916. Owners: Czech Technical University in Prague, Faculty of Biomedical Engineering; Institute of Physiology of the Czech Acad. Sci., Prague.
Ošťádal M, Bačáková L, Doubková M, Eckhardt A, Knitlová J, Novotný T. Směs pro léčbu pes equinovarus a farmaceutický přípravek ji obsahující. (A mixture for the treatment of talipes equinovarus and a pharmaceutical composition comprising it.) Utility model, Industrial Property Office, Prague, Czech Republic; No. 34329, accepted 27. 08. 2020. Owners: Institute of Physiology, Czech Academy of Sciences – Bulovka Hospital – Krajská zdravotní, a.s.
Potocký Š, Kromka A, Ižák T, Rezek B, Sedmera D, Bačáková L, Kopová I: Kompozitní povlak pro kostní implantáty. (Composite coating for bone implants). Utility model, Industrial Property Office, Prague, Czech Rep.; approved 23. 10. 2018 under No. 32210. Owners: Institute of Physics, ASCR, v.v.i., Institute of Physiology, ASCR, v.v.i.
Stehlik S, Rezek B, Potocky S., Kromka A, Kopova I, Bacakova L, Slepicka P, Slepicková Kasálková N: Nanodiamantový povlak fluoropolymerní folie pro kostní implantáty (A nanodiamond coating of fluoropolymer film for bone implants). Utility model, Industrial Property Office, Prague, Czech Rep.; approved 23. 10. 2018 under No. 32211. Owners: Institute of Physics, ASCR, v.v.i., Institute of Physiology, ASCR, v.v.i., University of Chemistry and Technology Prague.
Matejka R, Stepanovska J, Rosina J, Kneppo P, Brynda E, Riedel T, Filova E, Travnickova M, Zarubova J, Riedelova Z. Kultivační komora pro stimulaci planárních vzorků decelularizovaného perikardu. (A cultivation chamber for stimulation of planar samples of decellularized pericardium). Utility model No. 30705, Industrial Property Office, Prague CZ, accepted: 30.05.2017. Owners: Czech Technical University in Prague, Faculty of Biomedical Engineering, Kladno, CZ; Institute of Macromolecular Chemistry, Czech Academy of Sciences, Prague 6, CZ; Institute of Physiology, Czech Academy of Sciences, Prague 4, CZ
Matejka R, Stepanovska J, Rosina J, Hruzova D, Zarubova J.: Systém pro rotační endotelizaci cévních protéz. (A system for rotating endothelialization of vascular prostheses.) Utility model No. 31066, Industrial Property Office, Prague, CZ, accepted 03.10.2017. Owners: National Center for Tissues and Cells, a.s.; Czech Technical University in Prague Faculty of Biomedical Engineering; Institute of Physiology, Czech Academy of Sciences
Matejka R, Stepanovska J, Rosina J, Hruzova D, Zarubova J, Filova E. Kultivační komora pro dynamickou kultivaci tubulárních struktur. (A cultivation chamber for dynamic cultivation of cells on tubular carriers.) Utility model No. 30441, Industrial Property Office, Prague, CZ, accepted 13.12.2016. Owners: National Center for Tissues and Cells, Brno, Starý Lískovec, CZ, Czech Technical University in Prague, Faculty of Biomedical Engineering, Kladno, CZ, Institute of Physiology, Czech Academy of Sciences, Prague 4, CZ
Matejka R, Prochazka V, Izak T, Stepanovska J, Kromka A, Travnickova M, Bacakova L. Kultivační komora pro in-vitro opticko-elektrické monitorování biologických kultur s impedančními opticky-transparentními diamantovými elektrodami. (A cultivation chamber for in-vitro optical-electrical monitoring of biological cultures with impedance optical-transparent diamond electrodes.) Utility model, Industrial Property Office, Prague, Czech Rep.; registered on 21. 12. 2016 under No. PUV 2016-33219, approved on 18. 05. 2017 under No. UV 30691. Owners: Institute of Physics, Acad. Sci. CR, Prague; Institute of Physiology, Acad. Sci. CR, Prague.
Kromka A, Potocky S, Domonkos M, Martinova L, Rysova M, Bacakova L, Matejka R, Filova E. Multifunkční samonosná diamantová porézní podložka pro kultivaci buněk. (Multifunctional self-supporting diamond porous cell culture pad.) Utility model No. 29864, application No. 2016-32491, accepted 11. 10. 2016, No. 29864. Industrial Property Office, Prague, Czech Rep.; Owners: Institute of Physics, ASCR, v.v.i., Institute of Physiology, ASCR, v.v.i., Institute for Nanomaterials, Advanced Technologies and Innovations, Technical University in Liberec, CR
Functional samples, prototypes
Tomšů, Júlia – Brož, Antonín – Bačáková, Lucie. Vložka do šestijamkové kultivační destičky pro pěstování buněčné kultury na rozhraní vzduch-kapalina. [Insert for a six-well culture plate for growing cell cultures at the air-liquid interface.] ID: FGU-2025-VKB01; 2025. Owner: Institute of Physiology, Czech Academy of Sciences.
Filová E, Matějka R, Steinerová M, Trávníčková M, Knitlová J, Musílková J, Bačáková L, Pražák Š, Koňařík M, Pirk J, Lodererová A, Honsová E, Riedel T, Kučerová J, Brynda E. Design recelularizované perikardiální náhrady chlopně. (Design of recellularized pericardium heart valve replacement). ID: FVZ-2018-Design recel. perikard. náhrady chlopně. Owners: Institute of Physiology, Czech Academy of Sciences; Czech Technical University in Prague, Faculty of Biomedical Engineering; Institute of Clinical and Experimental Medicine; Institute of Macromolecular Chemistry, Czech Academy of Sciences, 2019
Štěpanovská J, Matějka R, Bačáková L. Sestava pro elektrickou stimulaci buněčné kultury v kultivační desce. (Experimental setup for cell culture electrical stimulation in a culture plate). Functional sample, ID FGU-2018-VKB01. Owner: Institute of Physiology, ASCR, v.v.i.
Matějka R, Bačáková L, Filová E, Hrůzová D, Musílková J, Steinerová M, Trávníčková M, Zárubová J. Endotelizovaná cévní protéza. (Endothelialized vascular prosthesis). Funkční vzorek, ID FVZ-2017-Endotelizovaná cévní protéza. Owners: Institute of Physiology, Czech Academy of Sciences – Czech Technical University in Prague, Faculty of Biomedical Engineering – National Center for Tissues and Cells, a.s.
Sopuch T, Bačáková L, Suchý P, Rýdl J, Kudláčková R, Drahovzalová R, Bačáková M, Kružicová A, Masteiková R, Reichert B, Vinklárková L. Biokompatibilní kombinované matrice pro hojení ran. (Biocompatible combined matrix for wound healing), Prototype, 2016, ID Hcel NaT 2016V003, Owner: Holzbecher, Ltd., Bleaching & Dyeing in Zlic, 552 03 Ceska Skalice – Zlic, Czech Republic
Filova E, Brynda E, Riedel T, Houska M, Kaplan O, Musilkova J, Steinerova M, Bacakova L, Matejka R, Zarubova J, Hruzova D. Chemicky modifikovaná decelularizovaná cévní protéza připravená pro endotelizaci: Použití fibrinové sítě. (Chemically modified decellularized vascular prosthesis prepared for endothelialization: Use of fibrin assembly.) Functional sample, ID FVZ-2016-Endotelizace, 2016. Owner: Institute of Physiology, ASCR, v.v.i.
Sopuch T, Bačáková L, Suchý P, Rýdl J, Kudláčková R, Drahovzalová R, Bačáková M, Kružicová A, Masteiková R, Reichert B, Vinklárková L. Biocompatible combined matrix for wound healing, 2016, ID Hcel NaT 2016V003, Owner: Holzbecher, Ltd., Bleaching & Dyeing in Zlic, 552 03 Ceska Skalice – Zlic, Czech Republic
Sopuch T, Rýdl J, Drahovzalová R, Bačáková L, Brožková I, Wiener J. Cellulosic matrices – basic biocompatible materials, 2015. Owner: Holzbecher, Ltd., Bleaching & Dyeing in Zlic, 552 03 Ceska Skalice – Zlic, Czech Republic
Balík K, Sochor M, Sucharda Z, Suchý T, Bačáková L: Hybridní kompozitní materiál na bázi polyamidové vláknové výztuže a polydimethylsiloxanové matrice adované nano fosforečnanem vápenatým jako náhrada kostní tkáně. [Hybrid composite material based on polyamide fabric reinforcement and polydimethylsiloxane matrix added by nano hydroxyapatite as bone tissue replacement.] ID: USMH/220/8; 2010. Owners: Institute of Physiology, Czech Academy of Sciences; Institute of Rock Structure and Mechanics, Czech Academy of Sciences; Czech Technical University in Prague / Faculty of Mechanical Engineering.
Bačáková L: Plastové kroužky pro uchycení biomateriálů na dně kultivačních misek. [Plastic rings for fixing biomaterials to the bottom of cell culture dishes.] ID: Plastové kroužky; 2007. Owner: Institute of Physiology, Czech Academy of Sciences
Bačáková L: Zjednodušená trypsinizace buněk. [A simplified trypsinisation of cells.] ID: Zjednodušená trypsinizace; 2007. Owner: Institute of Physiology, Czech Academy of Sciences.
Bačáková, Lucie: Standardizovaný test pro hodnocení biocompatibility umělých materiálů.
[Standardized test for evaluation of biocompatibility of artificial materials.] ID: Standardizovaný test; 2007. Owner: Institute of Physiology, Czech Academy of Sciences.
Verified technology
Bartoš Michal, Vančurová Marta, Bačáková Lucie, Baudyš Karel, Choc Milan, Sopuch Tomáš, Švorčík Václav. Sole oxidované celulózy pro finální formulace, resorbovatelné gely. Analytické metody pro hodnocení kvality produktu a jeho stability. (Salts of oxidited cellulose for final formulations, resorbable gels. Analytical metods for evaluation of product quality and its stability.) ID: Typová řada OKCEL. Owner: Synthesia, a.s.