The findings of scientists from the Department of Mitochondrial Physiology could be used for studies of type 2 diabetes developing and to determine the diagnosis of pre-diabetic conditions.
The islets of Langerhans in the pancreas contain β-cells that secrete insulin when stimulated by food, especially glucose, fatty acids or the peptide GLP1 secreted during digestion in the intestines. Disruption of these mechanisms, usually accompanied by insulin receptor dysfunction and impaired cellular response of peripheral tissues, especially muscle, adipose tissue and liver, leads to type 2 diabetes. Mitochondria act as glucose sensors in β-cells. Upon glucose uptake, mitochondria increase respiration and the synthesis of ATP, the substance that supplies all cells with energy. ATP then triggers processes on the plasma membrane of β-cells leading to the secretion of insulin vesicles. This secretion was known to be triggered by fatty acids, but the mechanism was unknown.
The researchers at the Department of Mitochondrial Physiology FGÚ AV ČR, under the direction of Dr. Petr Ježek, DrSc., studied the interactions of fatty acids with model β-cells in tissue culture, in which they switched off the studied genes, and thus determined their function. They also looked at how this affects insulin secretion in response to various stimuli, cell energetics and the production of reactive oxygen compounds, which are sometimes undesirable by-products. In healthy cells, however, they represent specific signalling means for various cell functions.
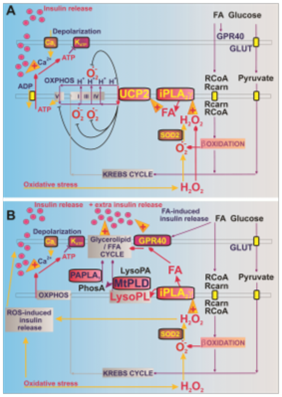
The enzyme that cleaves fatty acids from mitochondrial membranes, called phospholipase iPLA2γ, is directly activated by one of the signal reactive oxygen compounds, hydrogen peroxide H2O2. After this activation, phospholipase iPLA2γ cleaves fatty acids, which diffuse to the surface of β-cells where GPR40-type receptors are located. These receptors are activated upon interaction with fatty acids and trigger a cascade that leads to insulin secretion (see figure). Activation of the receptors by blood-derived fatty acids alone is not efficient. Dr. Ježek’s results show that “signal amplification” is needed by multiplying fatty acids that are continuously burned by so-called β-oxidation in the mitochondria. It is β-oxidation that produces the signal hydrogen peroxide H2O2, which activates the phospholipase iPLA2γ. This induces further cleavage of fatty acids until their interaction with GPR40 outweighs their combustion.
These findings can be used to study the development of type 2 diabetes, i.e. the development of initial pathological dysfunctions, e.g. of β-cells, which much later leads to diagnosed diabetes. The findings could also lead to the diagnosis of pre-diabetic conditions, as one sign of developing pathology is oxidative stress in β-cells.
Pic. A Protection against oxidative stress is provided by the mitochondrial phospholipase iPLA2γ and the uncoupling protein UCP2
Pic. B Fatty acids (FAs) do not stimulate the GPR40 receptor on the surface of pancreatic β-cells directly but by burning FAs through β-oxidation, which generates the signaling molecule H2O2. This activates the enzyme phospholipase iPLA2γ which cleaves other MKs. Once these have taken over, stimulating through GPR40 receptors the cycle given by glycerolipid and MK metabolism and leading to insulin secretion by pancreatic β-cells
Ježek, Jan – Dlasková, Andrea – Zelenka, Jaroslav – Jabůrek, Martin – Ježek, Petr H2O2-Activated Mitochondrial Phospholipase iPLA2 gamma Prevents Lipotoxic Oxidative Stress in Synergy with UCP2, Amplifies Signaling via G-Protein-Coupled Receptor GPR40, and Regulates Insulin Secretion in Pancreatic beta-Cells. Antioxidants & Redox Signaling. Roč. 23, č. 12 (2015), s. 958-972. ISSN 1523-0864. IF: 7.407, 2014.